New Human Physiology | Paulev-Zubieta 2nd Edition
Chapter 2: Muscle and Cells Disorders
| HOME | PREFACE | TABLE OF CONTENTS | SYMBOLS | SECTION INFO | CONTRIBUTORS | LINKS | CONTACT US |
Highlights
Study_ObjectivesPrinciplesDefinitionsEssentials
PathophysiologyEquationsSelf-AssessmentAnswers
Further Reading
|
Chapter 2
|
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
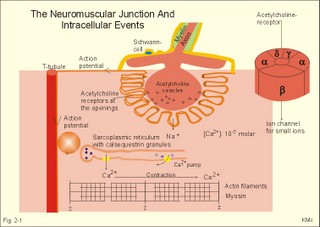
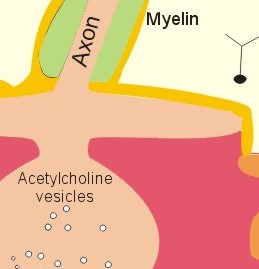
This paragraph deals with 1. Neuromuscular junctions, 2. Synapses, 3. Skeletal muscles, 4. Smooth muscles and 5. Cardiac muscle tissue. The neuromuscular endplate is the contact zone between the axons of motor neurons and striated muscle fibres. Axon terminals have vesicles containing acetylcholine (Fig. 2 -1). The vesicles dock on the active zones or release sites of the presynaptic membrane with high affinity. The muscle cell membrane at the endplate is folded in junctional folds or crypts (Fig. 2-1). Nicotinic acetylcholine receptors (Chapter 6) are concentrated at the openings of these junctional crypts. The release sites are located directly over the acetylcholine receptors (Fig. 2-1). The postsynaptic membrane has acetylcholinesterase all over its surface. The nicotinic acetylcholine receptor is related to a ligand (acetylcholine)-gated ion channel found not only in the neuromuscular junction, but also at all autonomic ganglia (Chapter 6) and in the central nervous system (CNS). The receptor is fixed into the postjunctional membrane, whereas acetylcholinesterase is loosely attached to its surface. The receptor has five integral protein subunits (2a , 1b , 1g , 1d ), surrounding a central ion channel pore that is opened by the binding of 2 acetylcholine molecules to the 2 a - proteins (Fig. 2-1). Opening of the ion channel increases the conductance for small cations (Na+ and K+) across the postjunctional membrane, depolarising the membrane potential of the cell. These ion channels are not voltage-gated (not dependent on changes in membrane potential), like most cation channels in neurons, cardiac and skeletal muscle cell membranes. Fig. 2-1: The neuromuscular junction and intracellular events. Acetylcholine = ACh. The ACh-receptor to the right is magnified. The acetylcholine-vesicles are probably already stored close to the release zones, awaiting the release signal (Fig. 2-1). When the action potential (AP) reaches the axon terminals, the axon membrane is depolarised, and voltage-gated Ca2+-channels are transiently activated. This causes Ca2+ to flow down its concentration gradient from the outside into the axon terminal. The influx of Ca2+ at the release zones causes the vesicles to fuse with the axon membrane, and empty acetylcholine into the 50 nm wide cleft by exocytosis (Fig. 2-1). After crossing the synaptic cleft by diffusion, acetylcholine binds to its receptor protein on the muscle cell membrane. This binding complex opens the ion channel and increases the conductance for small cations across the muscle cell membrane. The influxes of Na+ depolarise the endplate temporarily, the transient depolarization is termed the endplate potential (EPP). The EPP dies away when acetylcholine is hydrolysed to acetate and choline by the enzyme, acetylcholinesterase. The EPP has a large safety margin, as a single action potential in the motor axon will produce an EPP that always reaches the threshold potential in the muscle fibre. Rapid contraction of the muscle fibre is achieved by propagation of the muscle action potential along the whole length of the muscle fibre membrane and into the small, transverse tubules, which penetrate all the way through the muscle fibre (T-tubules in Fig. 2-1). The acetylcholine binding at the motor endplate increases endplate conductance and generates an action potential (AP) in all directions from the end plate (Fig. 2-1). The electrical excitation of the sarcolemma and the transverse tubules (T-tubules) during the AP triggers – by an unknown mechanism - the sarcoplasmic reticulum to release a pulse of Ca2+ (Fig. 2-1). The Ca2+-channels opens transiently in the vicinity of each sarcomere (Fig. 2-1). The sarcoplasmic [Ca2+] increases from 10-7 to 10-6 M (which is the threshold). This Ca2+ diffuses to the adjacent myofilaments, where they bind strongly to troponin C on the active filament, and end the troponin-tropomyosin blockade. This enables cyclic crossbridges to work as long as the high [Ca2+] is maintained, whereby contraction occurs. A continually active Ca2+-pump returns Ca2+ to the sarcoplasmic reticulum, and another Ca2+-pump in the cell membrane also reduces sarcoplasmic [ Ca2+] . Then the thin filament is off duty, because Ca2+ is withdrawn from its troponin C, the troponin-tropomyosin-blockade is re-established and relaxation ensues. The terminal cisternae of the sarcoplasmic reticulum contain granules of calsequestrin, a protein that can bind Ca2+ and reduce the concentration gradient (Fig. 2-1). Neurons with motor function have the ability to synthesise acetylcholine, because they contain choline-acetyltransferase. This enzyme catalyses the production of acetylcholine from acetyl-CoA and choline. Almost all cells produce acetyl-CoA and choline. Choline is also actively taken up from the extracellular fluid via a mechanism indirectly powered by the Na+-K+-pump. There is a 50% reuptake of choline from the synaptic cleft; hence some choline must be synthesized in the motor nerve. The postjunctional membrane depolarizes spontaneously - resulting in so-called miniature endplate potentials (MEP-potentials). A miniature endplate potential is probably caused by the spontaneous release of a single vesicle into the cleft. This is called quantal release. An endplate potential is prolonged when cholinesterase-inhibitors are present in the synaptic cleft. This is because these substances (eserine, edrophonium, malathion, parathion etc.) inhibits the enzyme and thereby protects acetylcholine from being hydrolysed by the enzyme. The life dangerous parathion poisoning is described in chapter 6. Under normal conditions, the endplate potential is terminated by the rapid hydrolysis of acetylcholine by acetyl-cholinesterase. Acetylcholine is a transmitter in the CNS, in all motor neurons, in all preganglionic neurons of the autonomic nervous system and postganglionic parasympathetic fibres, and in a few postganglionic sympathetic fibres. The cholinergic receptor subtypes are shown in Table 6-2. Chemical synapses prevail in humans, but we also have electrical synapses in gap junctions. A chemical synapse consists of a neuronal presynaptic terminal, a synaptic cleft and a subsynaptic (or postsynaptic) membrane with associated receptor proteins (Fig. 2-2). The chemical synapse is highly developed in the CNS. It conducts the signal one way only, and has a characteristic synaptic delay. The presynaptic axon terminal typically broadens to form a bouton terminaux (presynaptic terminal). 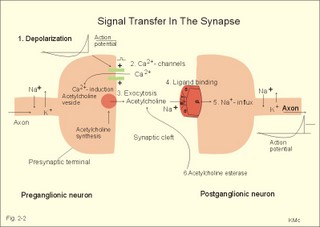
Fig. 2-2. A synapse between a preganglionic and a postganglionic neuron.
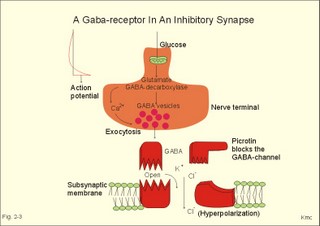
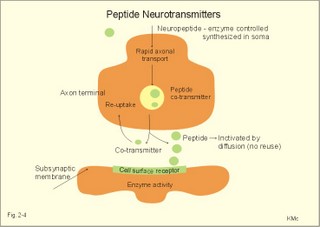
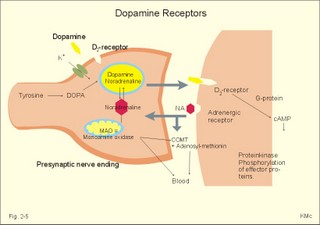
Influx of Na+ or efflux of K+ through the pores of such receptors changes the postsynaptic membrane potential. If the presynaptic action potential (AP) results in a postsynaptic depolarization, the transient is called an Excitatory Post-Synaptic Potential (EPSP). If the AP results in a postsynaptic hyperpolarization, the transient is called an Inhibitory Post-Synaptic Potential (IPSP). Excitatory synapses often use glutamate as the transmitter. The pores are penetrated mainly by Na+, which enters the cell, depolarizes the membrane, and produces an EPSP. The axon hillock on the cell body has a high density of voltage-gated Na+- and K+-channels. The axon hillock probably integrates the many synaptic potentials, and from here the action potential is generated. The dendrites have voltage-gated channels for K+ and for Ca2+. Recent evidence suggests that dendrites also contain voltage-gated Na+-channels, which are involved in electrogenesis (ie, movement of charge across the membrane). Each neuron in the CNS is in contact with up to 105 presynaptic axon terminals. Synaptic inputs are integrated at the axon hillock by either spatial or temporal summation. Spatial summation occurs when inputs from several axons arrive simultaneously at the same postsynaptic cell. Their postsynaptic potentials are additive. EPSPs summate and move the membrane potential closer to the threshold level for firing. Conversely, EPSPs and IPSPs cancel each other out. Temporal summation occurs when successive APs in a presynaptic neuron follow in rapid succession, so that the postsynaptic responses overlap and summate. Summation is possible because the synaptic potential lasts longer than action potentials by a factor of 10-100 times. Each individual synapse contains receptors, ion channels, and other key molecules, which are sensitive to the neurotransmitters released at the site. These specific protein molecules are involved in synaptic plasticity and summation. Electrical synapses. A gap junction is a transmembrane pathway of low electrical resistance that connects the cytoplasm of adjacent cells. A gap junction allows the membrane potential of the adjacent cells to be electrically coupled. Gap junctions form electrical synapses, which differ from chemical synapses in that transmission, is instantaneous. An electrical synapse consists of several protein pores, which close in response to increased intracellular [Ca2+] or [H+] in a cell, thereby increasing their resistance. Open gap junctions exchange ions and small molecules up to a molecular weight of 1000 Dalton. Gap junctions are found in simple reflex pathways, where rapid transfer of the electrical potential is essential, and between non-neural cells such as epithelial and myocardial cells, smooth muscle cells and hepatocytes. Neurotransmitters are divided into classical, rapidly acting non-peptides (Table 7-1) and putative, slowly acting neuropeptides (Table 7-2) - all dealt with in Chapter 7. Here is only described the function of GABA, neuropeptides and dopamine. The major inhibitory transmitters are GABA (gamma-aminobutyric acid) in the brain and glycine in the spinal cord. Binding of GABA to the GABA-receptor opens the pore for Cl- influx, whereby the subsynaptic cell membrane hyperpolarises (Fig. 2-3). The increase in Cl- conductance stabilises the membrane potential and decreases the efficacy of excitatory transmission. The GABA-receptor pore is permeable to K+ besides Cl-. The GABA-receptor has a major inhibitory role in brain function and is the binding site for barbiturates (used as hypnotics in anaesthesia) and for benzodiazepines (used to relieve anxiety). Fig. 2-3: A GABAA -receptor in an inhibitory synapse. The GABAA-receptor shown here is related to sedation and mood, whereas the GABAB-receptor controls spasticity (Chapter 7). Picrotin blocks the GABA-channel. Glutamate, aspartate and related acidic amino acids are the most important excitatory transmitters in the brain and spinal cord. Excitatory neurons possess excitatory amino acid (EAA) receptors. EAA receptors are a family of receptors with at least four different ions channels: The N-methyl-D-aspartate-receptor (NMDA), and three so-called non-NMDA receptors - one of which is the glutamate receptor. The NMDA-receptor operates with K+-efflux, while Na+ and Ca2+ enters the subsynaptic neuron. Mg2+ and many antiepileptic drugs block the NMDA-receptor channel (Chapter 7). Opening of Na+- and Ca2+-channels, which allow an increased influx of Na+ and Ca2+, cause the membrane potential to approach the threshold level for excitation. Both a reduced Cl--influx to the neuron and a reduced K+-efflux move the membrane potential towards the threshold level and possible excitation. The NMDA-receptor has a separate glycine site. Neuropeptides (Table 7-2) have slow excitatory or inhibitory transmitter actions. Peptides cannot be synthesized locally in the axon terminals, because they do not have ribosomes. Fig. 2-4: Peptide neurotransmitters Peptides are water soluble, and act as hormones by binding to specific cell-surface receptors. Cell-surface receptors are a family of guanosine triphosphate-binding proteins, so-called GTP-binding or G-proteins, which control and amplify the synthesis of second messengers. Cell-surface receptors for neurohormones can function as transport protein and possess enzyme activity (Fig. 2-4). Neuropeptides are build by a sequence of amino acids. Neuropeptides are synthesized in the cell bodies of the neurons and transported to the terminal buttons by rapid axonal transport (Fig. 2-4). Some neuropeptides are released together with a non-peptide co-transmitter (Table 7-2). Some neuropeptides are produced when a large mother-peptide is cleaved into several active neuropeptides. Neuropeptides are released from the nerve terminal near the surface of its target cell, and diffuse to the receptors of the target cell. Low concentrations of neuropeptides typically affect the membrane potential by changing the conductance of the target cell to small ions. The action of neuropeptides usually lasts longer than that of enzyme-inactivated transmitters. Following prolonged synaptic transmission, neuropeptides are deactivated by proteolysis. Dopamine and other catecholamines derive from tyrosine via DOPA, which stands for the precursor 3,4-dihydroxy-phenylalanine. Dopamine is the actively accumulated into storage vesicles in the nerve endings together with noradrenaline and ATP. Dopamine activates both presynaptic and subsynaptic D2-receptors (Fig. 2-5). Fig. 2-5: Dopamine receptors and the interactions with noradrenaline (NA). Noradrenaline can be oxidatively deaminated by monoamine oxidase (MAO) located on the external membrane of mitochondria (Fig. 2-5). The enzyme COMT (catechol-O-methyl transferase) can also methylate noradrenaline to nor-metanephrine. MAO and COMT are important in metabolising circulating catecholamines. Re-uptake of noradrenaline is the most important terminator of its actions. Activation of both D2-receptors opens K+-channels and the increased outflux of K+ hyperpolarizes the membrane. Blockage of the presynaptic D2-receptors in substantia nigra with antipsychotic drugs reduces K+-outflux and increases dopamine production and release. Loss of dopamine-containing neurons in substantia nigra results in the lack of dopamine at the D2-receptors of the striatal neurons. These neurons degenerate in Parkinson's disease causing muscular rigidity and hand tremor (Chapter 4). Skeletal or striated muscles are attached to a skeleton. Striated muscles are called striated, because they have a striking banding pattern. Microscopy with polarised light reveals dark (optically anisotropic) striations or A bands alternating with light or optically isotropic striations or I bands. Running along the axis of the muscle cell or muscle fibre is the myofibril bundles of filaments that are visible on electron micrographs. The A band contains the thick filaments of myosin, and the I band contains thin filaments of actin and tropomyosin (Fig. 2-6). The thin filaments are anchored to a transverse structure termed the Z disc (Fig. 2-6). Each contractile unit contains the halves of two I-bands with the A-band in between. This unit is a sarcomere. Sarcomeres have a length of 2.3-2.5 mm between the two Z discs at rest. The central A band is a relatively isotropic substance - also termed the H-band - with an M-line of darkly stained proteins that link the thick filaments into a fixed position. Contraction takes place by sliding of the filaments. The sliding of filaments against each other is called the sliding filament hypothesis, and since contraction works by cycling of millions of crossbridges, it is also called the theory of crossbridge cycling. The thin filaments are 1-1.2 mm long and consist of small globular proteins that form two helic pearl strings. The double helix of actin is supported by a long, thin molecule of tropomyosin that is situated along the groove of the double strands of actin (Fig 2-6). Each tropomyosin molecule interacts with 7 actin molecules on each side. Troponin is composed of 3 subunits: Troponin-C binds Ca2+, troponin-T reacts with tropomyosin, and troponin-I inhibits the actin-myosin-interaction, when Ca2+ is absent. Dystrophin is another normally occurring cytoskeletal muscle protein. The thick filaments are 1.6 mm long, and consist of large myosin molecules. Myosin is a dimer of almost 500 kD. Each monomer consists of one heavy chain and two light chains. The heavy chain consists of a helical tail and a globular head (Fig. 2-6). The light chains are associated with the head of the heavy chain. Since myosin is a dimer, the double-helix tail must end in two globular heads (Fig. 2-6). The globular heads contain the ATPase activity and the actin-binding site. The light chains control the rate of cross bridge cycling. 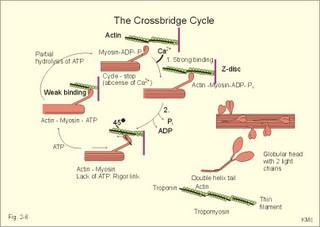
Fig. 2-6: Thick and thin filaments. The crossbridge cycle. The crossbridge cycle theory states that there are multiple cycles of myosin-head attachment and detachment to actin during a muscle contraction. When myosin binds to actin, an actinomyosin complex is formed - with an extremely active ATPase. The interaction between actin and myosin and the hydrolysis of ATP is the basic process that converts chemical energy into mechanical energy. Each crossbridge consists of two heads. At rest the crossbridge from myosin is not attached to actin. The globular myosin heads are oriented perpendicular to the filament axis (Fig. 2-6), and they have a high standard affinity for actin.
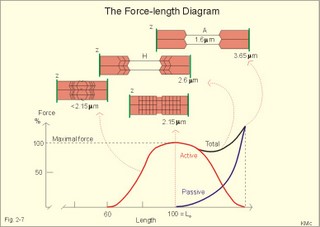
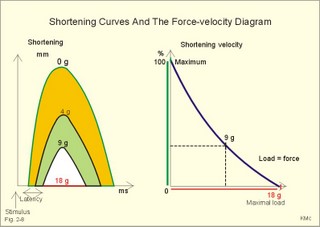
Fig. 2-7: Force-length diagram Force is required to stretch a relaxed muscle, because muscle tissue is elastic, and the force increases with increasing muscle length (Fig. 2-7). The passive blue curve reflects the properties of the elastic, connective tissue, which becomes less compliant or stiffer with lengthening (Fig. 2-7). A muscle contraction at constant length is termed isometric. Force is measured in Newton (N), and one N is the force required to accelerate a mass of one kg with an acceleration of one m s-2. In muscles, the traditional expression for force is stress or tension in N per cross-sectional area of the muscle (N m-2), which is actually pressure (Pascal, Pa). Here, the ordinate is force expressed as a percentage of the maximal force (Fig. 2-7). 1. The length at which maximum active contractile force is developed is called Lo, corresponding to a sarcomere length of 2.15 m m (Fig. 2-7). Lo is the length of the muscle in the body when at rest. At this length there is a maximum number of active crossbridges (Fig. 2-7). When an isolated muscle in an isometric force or stress-meter is stimulated, the active muscle force decreases with the decrease in overlap between thin and thick filaments; at a sarcomere length of 3.65 m m the isometric force reaches zero (Fig. 2-7). The force is always proportional to the number of cycling cross-bridges interacting with the thin filament. 2. Force also declines at muscle lengths less than Lo (Fig. 2-7). Thin filaments overlapping, and thick filaments colliding against Z-discs cause this. The isometric force (stress) decreases as the sarcomere length is reduced, as shown with the sarcomere length of less than 2.15 m m (Fig. 2-7). 3. When the active muscle length is stretched beyond any overlapping between the thin and the thick filaments the muscle can only develop a force of zero (see the sarcomere length of 3.65 m m with an A-band of 1.6 m m in Fig. 2-7). The lengths of the thick and thin filaments of human striated muscles are similar (1.6 and 1.2 m m, respectively). They generate maximal tension forces at Lo, corresponding to a sarcomere length of 2.2 m m, namely 300 kN per m2 or kPa. Muscle power or work-rate (Eq. 2-1) is the product of muscle force (N) and shortening velocity (m s-1). The maximal work rate of human muscles is reached at a contraction velocity of 2.5 m s-1. The maximal work-rate is thus (300 kPa *2.5 m s-1) = 750 kW per square meter of cross sectional area. Hill developed an equation for the shortening velocity of isotonic muscle contractions (Eq. 2-2). The equation is illustrated in Hills force-velocity diagram (Fig. 2-8). The maximum force is developed at the initial length (Fig. 2-8 right: 18 g of load). At 18 g there is no shortening – the length is unchanged. Stimulation of the unloaded muscle results in maximum shortening velocity (100%). An unloaded crossbridge can cycle at maximal rate, indicated by maximal shortening velocity (Fig. 2-8 right). The shortening velocity decreases rapidly as the afterload is increased describing a hyperbola (Fig. 2-8 right). With increasing loads the latency is increased and the shortening is reduced (4 and 9 g in Fig. 2-8 left). The latency depends on the length of thepreceding isometric phase. The maximal velocity of shortening is directly proportional to the myosin ATPase activity. We increase the velocity of muscle shortening under a given load by the recruitment of additional motor units. The long human arm muscles shorten at a rate of 8 m per s. Muscles can bear a load of 1.6 times the maximal force before the crossbridges are broken, but under such extreme conditions the work-rate (power) of the muscle approach zero (no shortening in Fig. 2-8 left). This is also the case when a person attempts to lift a motor car - the speed of shortening is zero (isometric contraction). On the other hand, the speed at which a pocket thief operates is probably impressive, although the force is minimal. Maximal work-rate occurs at a load of 1/3 of the maximal isometric force of the muscle. Here the contractile system has optimal efficiency in converting chemical energy into mechanical energy. Fig. 2-8: Hill's force-velocity diagrams (right) and related shortening curves (left). A further rise in filament velocity seems to reduce the potential for actin-myosin interaction. The crossbridge cycling rate falls as the load on the crossbridges increases (Fig. 2-8 right). In a muscle, the force of contraction is graded by increasing the frequency of action potentials, and by recruiting more muscle cells. Prolonged crossbridge contraction results in physiological tetanus. This is a prolonged muscle contraction maintained by the prolonged Ca2+-influx caused by repetitive stimulation. Human skeletal muscles consist of three functional types of motor units. A motor unit is a motor neuron with the muscle fibres it innervates. All muscle fibres belonging to a motor unit are of the same type. The three types of muscle fibres are characterised in Table 2-1.
Most human skeletal muscles are a mixture of all three types of motor units, although the proportions vary considerably. Type I: The slow motor units contain slow-oxidative (SO) red slow-twitch fibres.They are adapted to continuous postural muscle activity. The fibres have many mitochondria and a high content of myoglobin (red fibres). They depend on aerobic metabolism and the glycogen content is high. Slow motor units have weak but long lasting contractions (slow reaction to a signal or twitch). The fibres are small and are first to be recruited. During light work these highly excitable motor units activate red fibres suited for prolonged activity or endurance activities. Endurance training increases the oxidative capacity of the activated motor units, whereas strength training increases cellular hypertrophy. Type IIA: Fast-twitch, fatigue-resistant (FR) motor units have type IIA twitch fibres with a high or intermediate content of mitochondria, myoglobin, and glycogen. These fibres also rely upon oxidative metabolism (fast oxidative glycolytic = FOG) and have a high level of both oxidative and glycolytic metabolism. The motor units provide contractions of intermediate force and duration, and they resist fatigue. FOG fibres are of intermediate size, and they are recruited before the white fibres. This is in accordance with the size recruitment principle: Small or intermediate motor units are easier to activate by excitatory postsynaptic potentials (EPSPs) than large neurons. Type IIB: Fast-twitch fatiguable (FF) motor units produce fast contractions (fast-twitch), and fatigue easily, as the name implies. Their large white fibres, with their dense sarcoplasmic reticuli, are adapted to activities requiring large forces with rapid control of contraction and relaxation. The fast-twitch white fibres (also called type IIB due to the highest shortening velocity) have few mitochondria, small amounts of myoglobin (white fibres), and depend on glycolysis (high anaerobic metabolism). They have only small amounts of glycogen (fast glycolytic = FG). The FF motor neuron is large, the axon is thick and it branches so greatly that the FF motor unit innervates more muscle fibres. This is why FF motor units are capable of powerful contractions. The cell body receives type Ia afferents. The FF units are recruited last and mainly during maximal efforts such as sprinting. The production of ATP by glycolysis matches the high rate of ATP consumption. We have three major metabolic sources of ATP:
The same molecules as in striated muscle essentially cause contraction in smooth muscle, but the intracellular organisation and the dynamic characteristics are entirely different (Table 2-2).
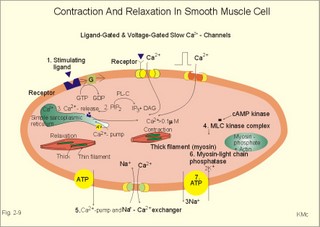
Smooth muscles are called so because they lack the distinct sarcomeric bands of striated muscles. Smooth muscle cells are spindle-shaped and line the hollow organs and the vascular system; the smooth muscle cells are extremely small (Table 2-2). Smooth muscle cells contain a few thick myosin-filaments, and many thin actin-filaments attached to dense bodies by a-actin (helical sarcomers). The cells are without regular sarcomers, Z disc's, myofibrils and T-tubules. Smooth muscle cells lack troponin. Dense bodies are analogous to Z disc's, and some dense areas are attached to the cell membrane. Smooth muscle cells do not contain a typical sarcoplasmic reticulum, which can store and release Ca2+. Instead some fibres possess an analogous simple reticular system located near the caveoli of the cell membrane. Caveoli are small invaginations of the membrane, similar to the T-tubules of striated muscles. The more extensive the reticular system is in the smooth muscle fibre, the higher is its shortening velocity due to release of Ca2+ mediated by IP3. Smooth muscle cells maintain large forces almost continually at extremely low energy costs. The same tension or tone is maintained for days in smooth muscle organs (intestine, urinary bladder, gall bladder) and can be obtained in striated muscle at high energy cost (up to 300 times the smooth muscle rate of ATP consumption). Smooth muscle cells are extremely sensitive to extracellular [Ca2+]. During an action potential the inward flux of ions is not Na+, but Ca2+ through slow Ca2+-channels. They open mainly in response to a ligand binding, but we have also voltage-dependent Ca2+-channels. The force-length relation is qualitative similar to that of striated muscles, so the sliding-filament mechanism is probably analogous (Fig. 2-6). The smooth muscle mechanism is special, because stimulation results in a maintained isometric force with strongly reduced velocities. Smooth muscle contractions are extremely slow. Ca2+ probably regulates the number of active crossbridges in smooth muscle slowly and indirectly. Smooth muscle cells contain some mitochondria, and they show a slow contraction pattern superimposed on the lasting tonus. Smooth muscle contractions typically last for 3 s, in contrast to striated muscle with total contraction periods of 10-100 ms. Since the energy demand in smooth muscle is extremely low, it is balanced by the oxidative ATP synthesis. Smooth muscle cells do not have an oxygen debt as striated muscles do, although they produce large amounts of lactate. This is probably because the ATP-synthesising glycolytic mechanism is located in the cell membrane and is linked to the ATP-utilising Na+-K+-pump. Smooth muscle contains far fewer myosin filaments than striated muscle. The myosin crossbridge heads of smooth muscle contain an isoenzyme with much less ATPase activity than that of striated muscle. Ca2+-entry through the cell membrane is much slower than internal release of Ca2+. A contracting smooth muscle fibre releases Ca2+ from two pools. The large extracellular fluid pool is essential. In the fibre that possesses a sarcoplasmic reticulum similar to the sarcoplasmic reticulum of striated muscle, there is a fast intracellular pool. The smooth muscle cell membrane contains a 3Na+-2K+-pump, a delayed K+-channel, a ligand-activated and a voltage-dependent Ca2+-channel, a sarcolemmal Ca2+-pump, and a Na+- Ca2+-exchanger (Fig. 2-9).
Fig. 2-9: Contraction and relaxation in smooth muscle cells. The ligand is acetylcholine in visceral cells and noradrenaline, ATP and peptide hormones in vascular smooth muscle cells.
When the high intracellular [Ca2+] during an action potential is lowered again towards the resting level, the cell relaxes. This is accomplished by stimulation of the sarcolemmal Ca2+-pump, and by blockade of both Ca2+-input and Ca2+-release. Metarterioles and precapillary sphincters without nerve fibres can still respond to the needs of the tissue by the action of local tissue vasodilatators. The following factors cause smooth muscle relaxation, and therefore vasodilatation: Adenosine, NO, lack of oxygen, excess CO2, increased [H+], increased [K+], diminished [Ca2+], and increased [lactate]. Endothelial-derived relaxing factor (EDRF) is recently shown to be nitric oxide (NO). Activation of endothelial cells produces NO from arginine, and NO diffuses into the smooth muscle cells. NO stimulates directly the enzyme guanylate-cyclase, and by that intracellular [cGMP] elevates. Circulating acetylcholine contracts the arterial smooth muscles when bound to cholinergic receptors. Smooth muscle cells grow (hypertrophies) as a response to the needs of the body, and they also retain the capacity to divide. During hypertension the lamina media of the arterioles hypertrophies which increases the total peripheral vascular resistance in the systemic circulation. These topics are further developed in Chapter 9. During pregnancy the (single-unit, see below) smooth muscles of the myometrium are quiescent and contain few gap junctions under the influence of progesterone. At term the myometrium grows and the number of gap junctions increases, due to the high oestrogen concentration. Now the myometrium is well prepared for the co-ordinated contractions during parturition (see Chapter 29). Smooth muscle changes length without marked changes in tension. Initially, there is a high tension developed upon stretching; then the tension falls as the myosin and actin filaments are reorganised by slowly sliding against each other. A sudden expansion of the venous system with blood results in a sharp rise in pressure followed by a fall in pressure over minutes. The smooth muscle fibres in the walls of the venous system are highly compliant, because they have accepted a large blood volume without much rise in pressure (delayed compliance). Smooth muscle cells are frequently involved targets in diseases such as hypertension, stroke,asthma, and many gastrointestinal diseases.Smooth muscle cells can be divided into multi-unit smooth muscle and single-unit smooth muscle. 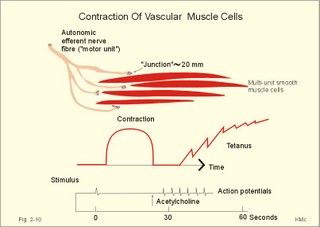
Fig. 2-10: Contraction of multi-unit smooth muscle cells (vascular). A single contraction is elicited by an electrical stimulus and later acetylcholine elicits tetanus. Contraction of multi-unit smooth muscle is controlled by extrinsic innervation or by hormones. Mechanical contact junctions between the cells are not found.
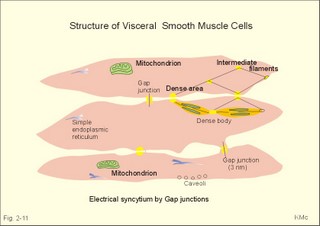
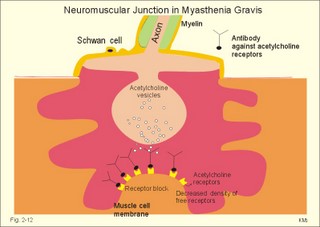
Fig. 2-11: Single-unit smooth muscle cells resemble cardiac muscle. Activity propagates from cell to cell through gap junctions forming an electrical syncytium. The dense bodies and dense areas contain alpha-actin. Action potentials generated in one cell can activate adjacent cells by ionic currents spreading rapidly over the whole organ and securing a co-ordinated contraction as though the tissue were a single unit or a syncytium. These cells are characterized by their spontaneous motility and by their sensitivity to stretch. The spontaneous activity is usually modified by the autonomic nervous system. Visceral smooth muscle undergoing peristalsis, generates propagating action potentials from cell to cell. Other cell-to-cell contacts are desmosome's and intermediary junctions subserving structural contact. These intermediary junctions transfer mechanical force from one smooth muscle cell to another on the plasma membrane, causing the single-unit smooth muscle cell to function like a stretch transducer. Myocardial cells are built of regular sarcomers just like the skeletal muscles, and they are contracting fast. Myocardial cells form an electrical syncytium in the same way as the single-unit smooth muscle cells. The characteristics of myocardial, skeletal and smooth muscle cells are presented in Table 2-2. Myocardial cells are mononuclear and the myoglobin, enzymatic and mitochondrial content are large just as the red fibres of skeletal muscles. The metabolism of myocardial cells is similar to that of red skeletal fibres, both being designed for endurance rather than speed and strength. The oxygen supply to the heart muscle must be maintained, if it is to synthesise ATP at a sufficient rate. Myocardial cells deprived of oxygen for 30-s cease to contract. Myocardial cells most resembles smooth muscle in its auto-rhythmicity and syncytial function. Pacemaker cells in the sinus node determine the normal cardiac frequency, because they send out spontaneous action potentials along the conduction system of the heart with a higher frequency than any other cells in the heart. Vagal stimulation releases acetylcholine at the pacemaker cells. Acetylcholine increases the K+-permeability, whereby K+ leaves the cell and hyperpolarizes the cell membrane. This is why the pacemaker (cardiac) frequency is reduced by vagal nerve stimulation. Sympathetic stimulation or adrenaline reduces the K+-permeability, so the depolarization is shortened, and the pacemaker frequency increased. The prolonged action potential characteristic for myocardial cells is initiated by an abrupt Na+-influx (phase 0) through fast Na+-channels just as in the striated muscles. The AP plateau is due to a slow Na+-Ca2+-channel, which deliver Ca2+ for the contraction activation. The action potential releases Ca2+ from the sarcoplasmic reticulum to the sarcoplasma. The effect is distributed by the cardiac T-tubule system. Cardiac contraction by crossbridge cycling depends on the presence of extracellular Ca2+ just as in smooth muscle tissue. Therefore, use of Ca2+-antagonists reduces the contractile force of the heart, whereas drugs, which increase Ca2+-permeability across the membrane, improve the contraction. In the heart, Ca2+-influx tends to prolong the depolarization just as in smooth muscle cells. The cardiac glycoside, digoxin, selectively binds to and inhibits the sarcolemmal 3Na+-2K+-pump, which leads to an increase in intracellular [ Na+] . Although the Na+-efflux is inhibited, the redundancy of Na+ affects the Na+-Ca2+-exchanger (3 Na+ out for one Ca2+ into the cell), leading to an increase in cellular [ Ca2+] and in the force of contraction. This is the mechanism of the increase in contractile force by digitalis glycosides. This paragraph deals with 1. Disorders of the neuromuscular junctions (myasthenia gravis), 2. Skeletal muscle disorders (dystrophia, dystonia, muscle injuries), 3. Smooth muscle disorders (asthma, hypertension etc) and 4. Myocardial disorders (coronary artery disease, arrhythmias, and chronic heart disease). 1. Disorder of the neuromuscular junction (Myasthenia gravis) This serious disease is acquired, but the cause is unknown. The development of this autoimmune disorder may be related to other diseases. Rheumatoid arthritis treated with D-penicillamine has resulted in myasthenia gravis. More than 50% of the myasthenia patients have thymic hyperplasia and some patients have a real thymoma. Many of these patients have an increased blood concentration of antibodies against their own acetylcholine receptor protein. There is a decreased density of receptor proteins on the postjunctional membrane. This was shown by the use of radioliganded toxins from poisonous snakes (which bind irreversibly to the acetylcholine receptor protein). The patients are tired and the muscles are extremely weak. This is particularly so for the proximal limb muscles, the extraocular muscles and the neck muscles, whereby the patient has difficulties in lifting the head. Mastication and swallowing is a difficult process. Fig. 2-12: Neuromuscular junction with antibodies and decreased density of acetylcholine-receptors in a patient with myasthenia gravis. As just mentioned the blood of most patients with myasthenia gravis contains autoantibodies against acetylcholine (ACh) receptor proteins on the cell surfaces of the motor end plates etc. The autoantibody competes for the ACh receptor and inhibits synaptic transmission, so muscular contraction is greatly inhibited. Deposition of immune complexes eventually destroys the ACh-receptor protein. Intravenous injection of an anticholinesterase improves the muscle strength immediately, but the beneficial effect is gone within 3 min. Thymectomy improves the condition and the prognosis also in the group of patients without thymoma. Oral anticholinesterase (such as pyridostigmine) has beneficial effect over 2-4 hours. They inhibit the enzyme acetylcholine-esterase, and thereby prolong the effect of naturally occurring acetylcholine on the receptors. In severe cases this treatment is inefficient, and immune-suppressants such as corticosteroids are sometimes favourable. Muscular dystrophy is an inherited disorder of skeletal muscles. Duchenne muscular dystrophy is an X-linked recessive muscle disorder characterized by the absence of dystrophin in the striated muscles and in the myocardium. The locus is localised to the Xp21 region of the X chromosome. Dystrophin is a normally occurring cytoskeletal muscle protein. The patient is a boy, who has to climb up his legs in order to reach the erect posture. Typically, there is proximal weakness with compensatory pseudohypertrophy of the calves. There is no cure and the patient dies from myocardial damage. Dystonias are prolonged muscle contractions leading to muscular spasms. There is a simultaneous action of opposing agonist and antagonist groups that produce abnormal postures. Dystonia is painful and particularly resistant to treatment. Dystonia musculorum deformans begins in childhood with generalized spasms that affect gait and posture. In most cases the cause is a genetic defect. Spasmodic torticollis causes the head to turn (torticollis) or change posture. Patients with a trigger zone on the jaw benefit from acupressure here. Muscle injuries are dealt with in Chapter 18. The most important disorders are asthma (Chapter 14) and systemic hypertension (Chapter 12). Smooth muscles are also involved in a disorder of swallowing (achalasia), where the myenteric plexus and the lower oesophageal sphincter fail to respond with receptive relaxation, and the food accumulates in the oesophagus. Other disorders of the gastro-intestinal smooth muscles are also treated there. 4. Disorders of the myocardium Coronary artery disorders (smooth muscles and myocardial disease) and congestive heart disease are treated in Chapter 10, and cardiac arrhythmias in Chapter 11. Only direct therapeutic uses of the systems developed till now are described here. Nitro-glycerine, nitroprusside and similar drugs relax smooth muscles by transfer of NO from endothelial cells. NO increases intracellular [cGMP] (Fig.11-1), which is the basis for the beneficial effect of the drugs on cardiac cramps. These second messengers activate protein kinases that phosphorylate effector proteins such as Ca2+-pumps and K+-channels. Such vasodilatators stimulate the sarcoplasmic Ca2+-pump, inhibit Ca2+-influx and stimulate K+-efflux through the delayed K+-channel (reduces the excitability). Hereby, the high intracellular [Ca2+] during an action potential is lowered towards the resting level (10-7 mM), and the smooth muscle cell relaxes producing vasodilatation. Eq. 2-1: Power (W) = Force (N) * Velocity (m s-1).
Eq. 2-2: Initial shortening velocity (v) = (Po - P)*b/(P + a) where P is the force or load acting on the muscle, Po is the maximal isometric force or load, a is a constant with the dimensions of a force, and b is a constant with the dimensions of velocity. I. Each of the following five statements have False/True options: A. Motor neurons synthesise acetylcholine unrelated to their content of choline-acetyltransferase. B. C. D. E. II. Each of the following five statements have False/True options:
Try to solve the problems before looking up the answers.
Kupfermann, I. "Functional studies of cotransmission." Physiol. Rev. 71: 683, 1991. Pollack, G.H. "Muscles and molecules: Uncovering the principles of biological motion." Seattle, Washington, 1990. Ebner & Sons. Alberts, B. et al. "Molecular biology of the cell." 4th Ed., 2002, Garland Publishing, Inc., New York & London.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Click here to introduce your comments or contributions