New Human Physiology | Paulev-Zubieta 2nd Edition
Chapter 28: Thyroid Hormones and Disorders
| HOME | PREFACE | TABLE OF CONTENTS | SYMBOLS | SECTION INFO | CONTRIBUTORS | LINKS | CONTACT US |
Highlights
Study_ObjectivesPrinciplesDefinitionsEssentials
PathophysiologyEquationsSelf-AssessmentAnswers
Further Reading
|
Chapter 28
|
 |
|
|
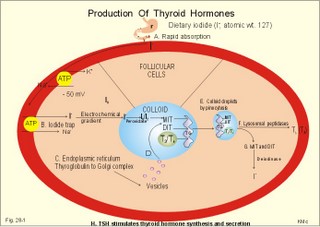
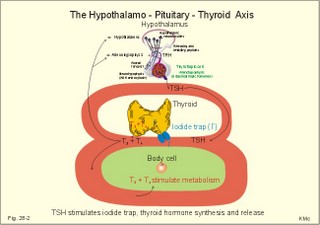
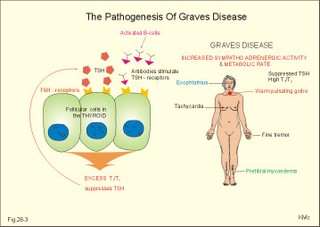
· To define concepts such as biological and physical half-life, cretinism, Graves disease, hypothyroidism, osteoporosis, PTH, TRH, TSH, thyrotoxicosis and struma. · To describe the synthesis of thyroid hormones, the iodine balance, the iodide trap, the endocytosis of colloid, the transport of thyroid hormone in plasma, · To draw a model of the functioning thyroid follicle. · To explain the thyroid hormone feedback control, the thyroid hormone metabolism, the mechanisms of effect of the thyroid hormones, and the tests of thyroid function. To explain hyperthyroidism, hypothyroidism, myxoedema, struma, iodine deficiency, cretinism, therapy of the disorders, and use of thyroid hormone in doping and obesity. · To use the above concepts in problem solving and case histories · The thyroid gland maintains the metabolic level of almost all cells in the body. · Thyroid hormones are essential for normal neural development, linear bone growth and proper sexual maturation. · Biological half-life refers to the rate of elimination of biologically active substances (hormones) by 50%. · Calcitonin is produced by the parafollicular C-cells of the thyroid. The hormone inhibits bone resorption by blocking the parathyroid hormone (PTH)-receptors on the osteoclasts. Calcitonin is important in bone remodelling and in treatment of osteoporosis. · Cretinism refers to a clinical condition caused by congenital hypothyroidism or infantile iodide deficiency. The clinical picture is a cretin or a mentally retarded hypothyroid dwarf. · Exophtalmus refers to bulging eyes - a sign, which is part of the thyroid eye disease. · Goitre (struma) is a visible or palpable enlargement of the thyroid gland. · Grave’s disease or Morbus Basedowii is the combination of thyrotoxicosis, struma and exophtalmus. · Hypothyroidism is an abnormally low activity of the thyroid gland with low circulating thyroid hormone levels caused by thyroid disease. · Myxoedema is a severe thyroid gland hypothyroidism in adults with a puffy swollen face due to a hard, non-pitting oedema called myxoedema or tortoise skin. · Osteoporosis (bone waste) is a term used for a marked reduction in all components of the bone mass. · Parathyroid hormone (PTH) refers to a single chain peptide hormone produced by the chief (C)-cells of the parathyroid glands. PTH accelerates osteolysis from bones, reduces the reabsorption of Ca2+ and phosphate from the proximal renal tubules and increases the reabsorption of Ca2+ from the distal tubules, and stimulates the renal production of biologically active vitamin D. · Thyroid releasing hormone (TRH) is released from the hypothalamus and reaches the adenohypophysis via the portal system. Here, the thyrotropic cells are stimulated to produce TSH. · Thyroid stimulating hormone (TSH) is released from the thyrotropic cells of the adenohypophysis to the systemic blood by which it travels to the thyroid gland. · Thyrotoxicosis (hyperthyroidism) is a condition probably caused by TSH-receptor antibodies, which bind to the thyroid follicle cells and stimulate the gland to secrete T3 and T4. The rise in thyroid hormone concentration will suppress TSH secretion. · Physical half-life refers to the rate of 50 % disintegration of radioactive isotopes. This paragraph deals with 1.The thyroid gland, 2. Synthesis and release of thyroid hormones, 3. Control of thyroid gland activity, 4. Metabolism of thyroid hormones, 5. Actions of thyroid hormones, and 6. Calcitonin. The thyroid gland maintains the metabolic level of almost all cells in the body by producing, in its follicular cells, two thyroid hormones: triiodothyronine (T3), and tetraiodothyronine (T4) or thyroxine. Iodine (I2) has an atomic weight of 127 and a molecular weight of 254; T4 has a molecular weight of 777 Daltons of which 508 is iodide. Thyroid hormones are essential for normal neural development, linear bone growth, and proper sexual maturation. Parafollicular cells called C-cells are located close to the follicular cells. C-cells produce the polypeptide hormone, calcitonin. 2. Synthesis and release of thyroid hormones Thyroid hormones are synthesised in adults as long as the dietary iodine (I2) supersedes 75 mg daily. This is an adequate supply to prevent goitre formation. The daily ingestion of iodide is 400-500 mg daily in many areas and the same amount is excreted in the urine in a steady state. The synthesis in the thyroid gland takes place in the following way: A. Dietary iodine (I2) is reduced to iodide (I-) in the stomach and gut is rapidly absorbed and circulates as iodide (Fig. 28-1). Fig. 28-1: The production and secretion of thyroid hormones. B. Follicular cells in the thyroid gland possess an active iodide trap that requires and concentrates iodide from the circulating blood (Fig. 28-1). Iodide is transported into the cell against an electrochemical gradient (more than 50 mV) by a Na+-I--symport. The iodide pump is linked to a Na+-K+-pump, which requires energy in the form of oxidative phosphorylation (ATP) and is inhibited by ouabain. The thyroid absorption of iodide is also inhibited by negative ions (such as perchlorate, pertechnetate, thiocyanate and nitrate), because they compete with the iodide at the trap. In the follicular cell, iodide passes down its electrochemical gradient through the apical membrane and into the follicular colloid. Iodide is instantly oxidised – with hydrogen peroxide as oxidant - by a thyroid peroxidase to atomic or molecular iodine (I0 or I2) at the colloid surface of the apical membrane. Thiouracil and sulfonamides block this peroxidase. C. The rough endoplasmic reticulum synthesises a large storage molecule called thyroglobulin. This compound is build up by a long peptide chain with tyrosine units and a carbohydrate unit completed by the Golgi apparatus. Iodide-free thyroglobulin is transported in vesicles to the apical membrane, where they fuse with the membrane and finally release thyroglobulin at the apical membrane. D. At the apical membrane the oxidised iodide is attached to the tyrosine units (L-tyrosine) in thyroglobulin at one or two positions, forming the hormone precursors mono-iodotyrosine (MIT), and di-iodotyrosine (DIT), respectively. This and the following reactions are dependent on thyroid peroxidase in the presence of hydrogen peroxide -both located at the apical membrane. As MIT couples to DIT it produces tri-iodothyronine (3,5,3`-T3), whereas two DIT molecules form tetra-iodothyronine (T4), or thyroxine. These two molecules are the two thyroid hormones. Small amounts of the inactive reverse T3 (3,3`,5`- T3) is also synthesised. E. Each thyroglobulin molecule contains up to 4 residues of T4 and zero to one T3. Thyroglobulin is retrieved back into the follicular cell as colloid droplets by pinocytosis. Pseudopods engulf a pocket of colloid. These colloid droplets pass towards the basal membrane and fuse with lysosomes forming phagolysosomes. F. Lysosomal exopeptidases break the binding between thyroglobulin and T4 (or T3). Large quantities of T4 are released to the capillary blood. Only minor quantities of T3 are secreted from the thyroid gland. G. The proteolysis of thyroglobulin also releases MIT and DIT. These molecules are deiodinated by the enzyme deiodinase, whereby iodide can be reused into T4 or T3. Normally, only few intact thyroglobulin molecules leave the follicular cells. H. TSH stimulates almost all processes involved in thyroid hormone synthesis and secretion. 3. Control of thyroid gland activity The hypothalamic-pituitary-thyroid axis controls the thyroid gland function and growth. a. The production and release of thyroid hormone is controlled by thyroid-releasing hormone (TRH) from the hypothalamus (Fig. 28-2). TRH reaches the anterior pituitary via the portal system, where the thyrotropic cells are stimulated to produce thyroid-stimulating hormone (TSH) or thyrotropin. TSH is the only known regulator of thyroid hormone secretion in humans. TSH is released to the systemic blood, by which it travels to the thyroid gland (Fig. 28-2). Here, TSH stimulates the uptake of iodide, and all other processes that promote formation and release of T4 (and T3). TSH activates adenylcyclase bound to the cell membranes of the follicular cells and increases their cAMP. T3 has a strong inhibitory effect on TRH secretion, as well as on the expression of the gene for the TRH precursor. Fig. 28-2: The negative feedback control of the hypothalamic-pituitary-thyroid axis. b. Almost all circulating T3 is derived from T4. TSH also stimulates the conversion of T4 to the more biologically active T3. Most of the circulating thyroid hormones are bound to plasma proteins, whereby the hormone is protected during transport. There is an equilibrium between the pool of protein-bound thyroid hormone and the free, biologically active forms (T3 and T4) that can enter the body cells. Thyroid hormones are lipid-soluble and they can easily cross the cellular membrane by diffusion. c. Inside the cell, T3 binds to nuclear receptors and stimulates cellular metabolism and increases metabolic rate. d. The concentrations of T3 and T4 in the blood are recorded by pituitary and hypothalamic receptors. This negative feedback system keeps the blood concentrations within normal limits, and there is only a minimal nocturnal increase in TSH secretion and T4 release. 4. Metabolism of thyroid hormones In the blood we have only small amounts of thyroxine-binding globulin (TBG; approximately 10 mg per l), but the affinity for T4 is high. The total T4 is 10-7 mol per l equal to 77.7 mg per l of blood serum, because 777 g of T4 equals one mol. out of the total. Approximately 70% of T4 and T3 binds to TBG, and the rest to thyroxine-binding albumin (TBA) and to transthyrenin. Oestrogens stimulate the synthesis of TBG. The T3 hormone is eliminated quickly (half-life: 24 hours), because it has the lowest degree of protein binding. The thyroxine (T4) molecule has a biological half-life of 7 days, almost equal to the physical half-life of the radioactive isotope 131I (8 days). T4 is likely to be a prohormone, which is deiodinised by monodeiodinase to the more potent T3 just before it is used in the cells. Thus T3 is probably the final active hormone, although it is present only in a very low concentration (10-9 mol per l). Most of the daily T4 released from the thyroid gland undergoes deiodination, with subsequent deamination and decarboxylation. Some of the hormone molecules are coupled to sulphate and glucuronic acid in the liver and are excreted in the bile. In the intestine most of the coupled molecules are hydrolysed, and the hormones are reabsorbed by the blood, whereby they reach hepar again (the enterohepatic circuit). 5. Actions of thyroid hormones Thyroid hormones are lipid-soluble and pass through cell membranes easily. T3 binds to specific nuclear receptor proteins with an affinity that is tenfold greater than the affinity for T4. The information alters DNA transcription into mRNA, and the information is eventually translated into many effector proteins. One type of thyroid receptor protein is bound to thyroid regulatory elements in target cell genes. Important cellular constituents are stimulated by T3: The mitochondria, the Na+-K+-pump, myosin ATPase, adrenergic b-receptors, many enzyme systems and proteins for growth and maturation including CNS development. Thyroid hormones stimulate oxygen consumption in almost all cells. Thyroid hormones stimulate the rate of 1) hepatic glucose output and peripheral glucose utilisation, 2) hepatic metabolism of fatty acids, cholesterol and triglycerides, 3) the synthesis of important proteins (the Na+-K+-pump, respiratory enzymes, erythropoietin, b-adrenergic receptors, sex hormones, growth factors etc), 4) the absorption of carbohydrates in the intestine and the gut excretion of cholesterol, and 5) the modulation of reproductive function. The many rate-stimulating effects are summarized in an overall increase in oxygen consumption. This slow - but long lasting - calorigenic and thermogenic effect is confined to the mitochondria. The thyroid hormones and the catecholamines work together in metabolic acceleration. Thyroid hormones increase cardiac rate and output as well as ventilation. The high basal metabolic rate raises the core and shell temperature, so that the peripheral vessels dilatate. This vasodilatation forces the cardiac output to increase. A circulatory shock develops, if the rise in cardiac output is insufficient to match the vasodilatation - socalled high output failure. A human body overloaded with thyroid hormones for a prolonged period (hyperthyroidism) will suffer from muscle atrophia, bone destruction and hunger damage, due to increased catabolism of cellular proteins and fat. Eventually hypothyroidism may develop due to suppression. is produced by the parafollicular C-cells of the thyroid. Calcitonin inhibits bone resorption by blocking the parathyroid hormone (PTH)-receptors on the osteoclasts. The result is an extremely effective lowering of plasma-[Ca2+ ] and -[phosphate]. Calcitonin is important in bone remodelling and in treatment of osteoporosis. Calcitonin is a single-chain peptide with a disulphide ring, containing 32 amino acids. Calcitonin is secreted from the thyroid gland in response to hypercalcaemia and it acts to lower plasma [Ca2+], as opposed to the effect of PTH. Administration of calcitonin leads to a rapid fall in plasma [Ca2+]. Calcitonin is the physiologic antagonist to PTH and inhibits Ca2+ -liberation from bone (ie, inhibits both osteolysis by osteocytes and bone resorption by osteoclasts). But calcitonin reduces plasma phosphate just as PTH. Calcitonin probably inhibits reabsorption of phosphate in the distal tubules of the kidney, but calcitonin also inhibits the renal reabsorption of Ca2+, Na+ and Mg2+. Calcitonin may inhibit gut absorption of Ca2+ and promote phosphate entrance into bone and cause important bone remodelling. Calcitonin deficiency does not lead to hypercalcaemia, and excess calcitonin from tumours does not lead to hypocalcaemia. Therefore, most effects of calcitonin are evidently offset by appropriate regulation through the actions of PTH and vitamin D. Calcitonin in plasma declines with age and is lower in women than in men. Low levels of calcitonin are involved in accelerated bone loss with age and after menopause (osteoporosis). Calcitonin protects the female skeleton from the drain of Ca2+ during pregnancy and lactation. Calcitonin is a neurotransmitter in the hypothalamus and in other CNS locations. Calcitonin is administered to postmenopausal females in attempt to prevent osteoporosis. This paragraph deals with 1. Hyperthyroidism, 2. Hypothyroidism, 3. Struma, and 4. Thyroid medullary carcinoma. The classical hyperthyroidism or thyrotoxicosis (Graves thyroiditis, Basedows disease) is a condition characterized by an abnormal rise in basal metabolic rate, struma and eye signs (thyroid eye disease). The eyes of the patient typically bulge (ie, exophtalmus). Patients with thyrotoxicosis have overwhelmingly high metabolic rates. Thyroid eye disease (with exophtalmus) is not confined to Graves’s hyperthyroidism only. Some exophtalmus patients are euthyroid or hypothyroid. Common to all types of thyroid eye diseases are specific antibodies that cause inflammation of the retro-orbital tissue with swelling of the extraocular eye muscles, so they cannot move the eyes normally. Proptosis and lid lags are typical signs, and conjunctivitis and scars on the cornea follow due to lack of protective cover. The oedematous retro-orbital tissue may force the eye balls forward and press on the optic nerve to such an extent that vision is impaired or blindness results. The best treatment is to normalise the accompanying thyrotoxicosis. Other therapeutic measures are palliative. TSH receptor antibody (IgG antibodies) release causes Graves’s disease from activated B-cells (Fig. 28-3). A genetic deficiency is involved, which is shown by the 50% concordance in monozygotic twins. Trigger mechanisms are presumed to be bacterial or viral infections producing autoimmune phenomena in genetically deficient individuals. The autoimmune system can produce the following autoantibodies: 1. TSH-receptor antibodies to the TSH receptors (antigens) on the surface of the thyroid follicular cells, which they stimulate just like TSH itself, causing thyroid hypersecretion. These IgG antibodies are also termed long-acting thyroid stimulator. 2. Specific autoantibodies causing retro-orbital inflammation and thyroid eye disease. 3. Thyroglobin antibodies against the storage molecule, thyroglobin. 4. Microsomal antibodies against thyroid peroxidase. These autoantibodies can be found in the plasma of most cases of Grave’s disease. Fig. 28-3:The pathogenesis of Graves disease, and the clinical manifestations of Graves’s disease. The increased metabolic rate and sympatho-adrenergic activity dominate the patient. The patient is anxious with warm and sweaty skin, tachycardia, palpitations, fine finger tremor, and pretibial myxoedema (ie, accumulation of mucopolysaccharides - see Fig. 28-3). Typically is a symmetrical, warm pulsating goitre. Lean hyperthyroid females - like female distance runners - have small fat stores and greatly reduced menstrual bleedings (oligomenorrhoea) or even amenorrhoea. The high T3 level increases the density of b-adrenergic receptors on the myocardial cells. The cardiac output is high even at rest and arrhythmias are frequent (eg, atrial fibrillation). Elderly patients may present with an apathetic hyperthyroidism, where they complain of tiredness and somnolence. Measurement of serum TSH with T3/T4 reveals that the diagnosis is not hypo- but hyperthyroidism. Erroneous treatment with thyroid hormone can kill the patient by causing vasodilatation and cardiac output failure. A suppressed serum TSH confirms the diagnosis of hyperthyroidism, and the serum T3 or T4 is raised. Several drugs are used in the treatment of hyperthyroidism. Carbimazole and methimazole inhibit the production of thyroid hormone and have immuno-suppressive actions. Monovalent anions and ouabain inhibit the iodide trap. Thiocarbamide inhibits the iodination of tyrosyl residues. Sulphonamides inhibit thyroid peroxidase, which oxidises iodide to iodine. Large doses of iodide inhibit the TSH-receptors on the thyroid gland. The high activity of the sympatho-adrenergic system is inhibited by b-blockers, preferably with central sedative effects. Subtotal thyroidectomy is used to treat patients with a large goitre, or patients with severe side effects to drug therapy. Radioactive iodine is stored in the gland and destroys the follicle cells. This therapy is complicated, and some patients develop hypothyroidism. Toxic goitre and toxic solitary adenoma (Plummers disease) are cases of secondary hyperthyroidism just as inflammation in acute thyroiditis and chronic thyroiditis. The cells secrete thyroid hormone without inhibition from the hypothalamo-pituitary axis. Primary hypothyroidism is an abnormally low activity of the thyroid gland with low circulating thyroid hormone levels caused by thyroid disease. Secondary hypothyroidism results from hypothalamic-pituitary disease. Primary hypothyroidism is caused by microsomal autoantibodies precipitated in the glandular tissue. Lymphoid infiltration of the thyroid may eventually lead to atrophy with abnormally low production of T4. Another clinical form starts out as Hashimotos thyroiditis, often with hyperthyroidism and goitre. Following atrophy caused by microsomal autoantibodies, the condition ends as hypothyroidism, or the patient is euthyroid. When hypothyroidism is congenital both physical and mental development is impaired and cretinism is the result. Also iodide deficiency in childhood may also result in a cretin or a mentally retarded hypothyroid dwarf. Myxoedema in the adult is severe thyroid gland hypothyroidism with a puffy swollen face due to a hard, non-pitting oedema (called myxoedema or tortoise skin). The skin is dry and cold; there is bradycardia, often cardiomegaly (ie, myxoedema heart), hair loss, constipation, muscle weakness and anovulatory cycles in females. A high TSH level and a low total or free T4 in plasma confirms the diagnosis primary hypothyroidism. Thyroid autoantibodies are usually demonstrable in the plasma. Hypercholesterolaemia and increased concentrations of liver and muscle enzymes (aspartate transferase, creatine kinase) in the plasma is typical. As stated thyroid gland high TSH characterises hypothyroidism. A test dose of TSH to a patient with thyroid hypothyroidism will not stimulate the thyroid gland. A test dose of TRH will result in an increased TSH response in thyroid gland hypothyroidism and decrease in hyperthyroidism. This is due to the negative feedback of thyroid hormones on the hypophysis. Hypothyroid females often have excessive and frequent menstrual bleedings (menorrhagia and polymenorrhoea). Hypothyroid patients exhibit slow cardiac activity. Secondary hypothyroidism is caused by reduced TSH drive due to pituitary or hypothalamic insufficiency. A test dose of TRH to a myxoedema patient with hypothalamic or pituitary insufficiency will result in a normal TSH response. Replacement is given to the hypothyroid patient with approximately 100 mg T4 daily for the rest of the patients life. Struma is a visible or palpable enlargement of the thyroid. Struma is due to iodine deficiency, increased iodine demand or strumagens. Any prolonged TSH stimulation results in an enlarged thyroid. Diseases in the thyroid gland including struma are caused by malfunction in the gland itself or by hypothalamic-pituitary defects. 4. Thyroid medullary carcinoma Mutations of a gene located on chromosome 10 can produce an error in receptor tyrosine kinase proto-oncogene associated with thyroid medullary carcinoma. Each of the following five statements have True/False options: A. Mutations of a gene located on chromosome 10 can produce a change in receptor tyrosine kinase proto-oncogene associated with thyroid medullary carcinoma. B. Thyroid hormones are water-soluble, which is why they pass through cell membranes quite easily. C. Struma is due to iodine deficiency, increased iodine demand or strumagens. D. Calcitonin is secreted from the thyroid gland in response to hypercalcaemia and it acts to lower plasma [Ca2+] as opposed to the effect of PTH. E. Tri-iodothyronine has a strong stimulatory effect on TRH secretion. A female, 62 years of age, suffers from pernicious anaemia for which she has received 1 (one) mg cyanocobalamine intramuscularly every 3.month for the last 10 years. At a routine visit the patient is found with a puffy swollen face due to a non-pitting oedema. Her skin is dry and cold, the heart rate is 55 beats per min, her hair is sparse, and she complains of constipation and fatigue. A series of blood tests reveals the following: High levels of microsomal autoantibodies against the thyroid gland and autoantibodies against her parietal cells. The TSH concentration in the plasma is high, whereas the T4 is low. The haematological variables are satisfying. 1. What is the probable diagnosis? 2. What are the treatment? 3. Is there any connection between pernicious anaemia and the other condition? A 49-year-old female (weight 52 kg; height 1.69 m) is in hospital and is being examined for thyroid disease. Her distribution volume for iodine is 12 l and her renal clearance is 36 ml plasma per min. In a period where her iodine intake equals her output, she is subjected to the following test. In the morning she receives a small dose of the radioactive isotope 131I, and three (3) hours later she urinates. From that moment she collects her urine for the following two (2) hours. The urine collection has a volume of 0.2 l and an iodine concentration of 65 mg per l. The total urine radioactivity is 1.6×107 disintegration per s (Becquerel or Bq). During the two hour test period, her plasma concentration of 131I falls from 38 300 to 26 100 Bq per l. The radioactivity in the thyroid gland (measured with a scintillation counter) increases during the test by 77 500 Bq. 1. Calculate the concentration of iodide in her plasma at the start of the test and at the end of the test. 2. Calculate the uptake of iodide in the thyroid during the 2-hour test and compare the result with a mean value of 2.4 mg per hour for healthy persons. 3. Calculate the thyroid plasma clearance for iodide and compare the result to the expected value of 10 ml per min. 4. Calculate the elimination rate constant for iodide. 5. Calculate the biological half-life for iodide in its distribution volume and compare the result to the physical half-life of 131I (8 days). See answers · T4 is likely to be a prohormone, which is deiodinised by monodeiodinase to the more potent T3 just before it is used in the cells. Thus T3 is probably the final hormone, although it is present only in a very low concentration (10-9 mol per l). · Thyroid hormones are synthesised in adult persons as long as the dietary iodine (I2) supersedes 75 mg daily. This is an adequate supply to prevent goitre formation. · The endoplasmic reticulum synthesises a large storage molecule called thyroglobulin. This compound is build up by a long peptide chain with tyrosine units and a carbohydrate unit completed by the Golgi apparatus. Iodine-free thyroglobulin is transported in vesicles to the apical membrane, where they fuse with the membrane and finally release thyroglobulin at the apical membrane. · Thyroid hormones stimulate oxygen consumption in almost all cells. They stimulate the rate of 1) hepatic glucose output and peripheral glucose utilisation, 2) hepatic metabolism of fatty acids, cholesterol and triglycerides, and 3) the synthesis of important proteins. The many rate-stimulating effects are summarized in an overall increase in oxygen consumption. This slow - but long lasting - calorigenic and thermogenic effect is confined to the mitochondria. · The thyroid hormones and the catecholamines work together in metabolic acceleration. Thyroid hormones increase the number of b-adrenergic receptors. Thyroid hormones modulate the secretion of sex hormones (sex development), growth hormone (growth), and nerve growth factors (CNS development). · The high basal metabolic rate raises the core and shell temperature, so that the peripheral vessels dilatate. This vasodilatation forces the cardiac output to increase. A circulatory shock develops, if the rise in cardiac output is insufficient - so-called high output failure. · Calcitonin is produced by the parafollicular C-cells of the thyroid. Calcitonin inhibits bone resorption by blocking the PTH receptors on the osteoclasts. The result is an extremely effective lowering of plasma [Ca2+ ] and [phosphate]. Calcitonin is important in bone remodelling and in treatment of osteoporosis. · The classical hyperthyroidism or thyrotoxicosis (Graves thyroiditis, Basedows disease) is a condition characterized by an abnormal rise in basal metabolic rate, struma and eye signs (thyroid eye disease). The eyes of the patient typically bulge (ie, exophtalmus). Patients with thyrotoxicosis have overwhelmingly high metabolic rates. · Primary hypothyroidism is abnormally low activity of the thyroid gland with low circulating thyroid hormone levels caused by thyroid disease. Secondary hypothyroidism results from hypothalamic-pituitary disease. · Primary hypothyroidism is caused by microsomal autoantibodies precipitated in the glandular tissue. Lymphoid infiltration of the thyroid may eventually lead to atrophy with abnormally low production of T4. Another clinical form starts out as Hashimotos thyroiditis, often with hyperthyroidism and goitre. Following atrophy caused by microsomal autoantibodies, the condition ends as hypothyroidism, or the patient is euthyroid. When hypothyroidism is congenital both physical and mental development is impaired and cretinism is the result. Also iodide deficiency in childhood may result in a hypothyroid dwarf or cretin. · Myxoedema in the adult is severe thyroid gland hypothyroidism with a puffy swollen face due to a hard, non-pitting oedema (tortoise skin called myxoedema). The skin is dry and cold; there is bradycardia, often cardiomegaly (ie, myxoedema heart), hair loss, constipation, muscle weakness and anovulatory cycles in females. · Struma is a visible or palpable enlargement of the thyroid. Struma is due to iodine deficiency, increased iodine demand or strumagens. Any prolonged TSH stimulation results in an enlarged thyroid. Griffin, J.E. and S.R. Ojeda. "Textbook of Endocrine Physiology." 5th Ed. Oxford University Press, N.Y./London, 2004. Hofstra, R.M.W. et al. "A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma." Nature 367: 375-377, 1994.
|
||
Click here to introduce your comments or contributions