New Human Physiology | Paulev-Zubieta 2nd Edition
Chapter 17 :The Acid-Base Balance and Disorders
| HOME | PREFACE | TABLE OF CONTENTS | SYMBOLS | SECTION INFO | CONTRIBUTORS | LINKS | CONTACT US |
Highlights
Study_ObjectivesPrinciplesDefinitionsEssentials
PathophysiologyEquationsSelf-AssessmentAnswers
Further Reading
|
Chapter 17
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
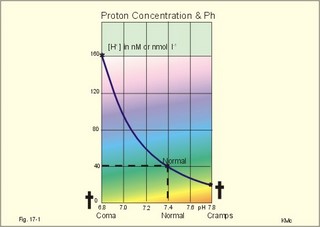
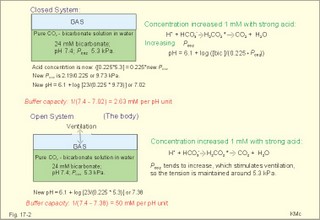
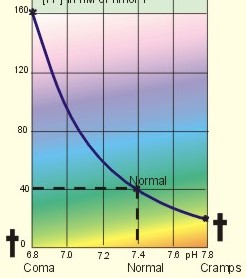
· To define pH, pK, PaCO2, acids, bases, buffers, buffer bases (titratable base), actual bicarbonate and non-carbonic buffers, the extended extracellular fluid volume (ECV extended with red cells), and the base excess of the extended ECV. · To describe the daily acid-base balance, titratable acidity and the net excretion of acid in the urine, the base excess of the blood and extended ECV, and the buffers of the intracellular fluid volume. · To calculate the third variable of the Henderson-Hasselbalch equation from two measured variables. · To draw the acid-base charts (ie, a double logarithmic plot of the Henderson-Hasselbalch equation). · To explain the acid-base chart, metabolic processes involved in the regulation of pH, tubular H+-secretion, and renal bicarbonate reabsorption. To describe intracellular buffers and pH, transport of H+ across the cell membrane. To describe four acute disorders of the acid-base status (primary respiratory and metabolic acidosis and alkalosis) and their chronic counterparts with therapeutic suggestions. · To use the acid-base variables in problem solving and diagnosis of acid-base disorders. · The mass action equation. Blood plasma is an open system where gasses are in equilibrium both with the alveolar air in the lungs and with the extracellular fluid volume (ECV). ECV and all red cells work as a buffer unit called extended ECV. Bicarbonate -carbonic acid is the strongest buffer and haemoglobin is the strongest non-carbonic buffer. The pH of arterial blood plasma is a function of PaCO2 and of the concentration of titratable buffer bases in the extended ECV (Table 17-1). The concentration of titratable base (Base Excess, BE) is measurable, and BE remains zero during acute changes of PaCO2. · The disorders of the acid-base balance are described by the following two interrelated chemical reactions: CO2 + H2O Û* H2CO3 Û H+ + HCO3- and [ Acid form] « H+ + [ Non-carbonic buffers-] Non-carbonic buffers - or non-bicarbonate buffers - refer to all other relevant buffers than the carbonic acid-bicarbonate buffer system. The enzyme carbo-anhydrase is shown with an asterisk: *. · Acid (HB) is defined as a compound that can release a hydrogen ion or proton (H+), whereas a base (B-) can bind H+. · Acidosis (acidaemia) is defined as a disorder with accumulation of acids in the extended ECV. The pH measured in the arterial blood is less than 7.35. · Alkalosis (alkalaemia) is defined as a condition with accumulation of bases in the extended ECV. The pH of the arterial blood is larger than 7.45. · Actual bicarbonate concentration is the concentration calculated from PaCO2 and pH measurements in the arterial blood sample. The value can be calculated with Eq. 17-6 or read from an acid-base chart with a bicarbonate axis (slope -1 in Fig. 17-3). · The acid-base buffer capacity of a system is defined as the amount of strong acid or base added to one litre (l) of the system (ie, mmol per l or mM) in order to change the pH one unit. · A buffer is a corresponding acid-base pair, which acts as a pH-stabiliser. · Buffer base (BB) refers to the total concentration of all carbonic buffer anions plus the non-carbonic anions. · Carbonic acid refers to carbon dioxide. · Carbonic buffers refer to the carbonic acid-bicarbonate buffer system. · Glomerular filtration is due to a hydrostatic/colloid osmotic pressure gradient - the Starling forces · The acid-base buffer capacity of the extended ECV is normally 61 mM per pH unit as an average (Table 17-1). · Non-carbonic buffers (non-bicarbonate buffers) refer to all other buffers than the carbonic acid-bicarbonate buffer system in the extended ECV (Table 17-1). · The Base Excess (BE) of the extracellular fluid (extended ECV) is calculated as the concentration difference of buffer bases in the actual sample and in the same sample following titration with strong acid or base and equilibration to standard conditions (pH 7.4, PaCO2 5.3 kPa or 40 mmHg). The difference is given in mmol of strong acid or base, which must be added to 1 l of the sample. The base excess remains normal (zero) in acute respiratory acid-base disorders · Extended extracellular fluid volume (extended ECV) is the extracellular fluid volume (ECV) plus the erythrocyte volume. A useful model of the extended ECV is an arterial blood sample diluted three folds with its own plasma (ie, 2+1). Extended ECV behave as a functional buffer unit. As a model extended ECV is considered to be all red cells distributed evenly in the extracellular fluid volume. The extended ECV of a healthy standard person covers approximately 20% of body weight. The principal buffers of the extended ECV in a healthy person are shown below (Table 17-1). Extended ECV serves as an early distribution volume in acid-base disorders. · Intracellular fluid volume (ICV minus red cells) comprises approximately 40% of the body volume. The principal buffers of ICV are proteins, phosphate and bicarbonate. The transport across cell membranes take hours to equilibrate and the intracellular buffer effect is involved in the delayed distribution. The intracellular buffer effect is larger in disorders with negative base excess (acidosis with pH approaching pK for the best buffers) than with positive base excess. OH- has a molecular weight 17 times larger than that of H+, so H+ diffuses faster through the cell membrane, since the diffusion rate depends on the hydrated ionic radius · pH is defined as the negative logarithm to the proton concentration. In this Chapter the pH is measured with glass electrodes in the arterial blood sample, and used in the extended ECV · pK is equal to pH, when the concentration of acid and of base is the same. · Partial pressure of carbon dioxide in the arterial blood sample (PaCO2) is measured with glass electrodes, and PaCO2 refers to the extended ECV in this Chapter. · Metabolic acidosis is acidosis with negative Base Excess. - Negative Base Excess means that the numeric difference between the measured buffer base (BB) and the normal buffer base (NBB) is negative. · Metabolic alkalosis is alkalosis with positive Base Excess. - Positive Base Excess means that the numeric difference between the measured buffer base (BB) and the normal buffer base (NBB) is positive · Metabolic acid-base disorders are caused by changes of the titratable base of the extended ECV (Base Excess is positive or negative). · Nephron - the functional renal unit. Each nephron consists of a glomerulus, a proximal convoluted tubule, a Henle loop and a distal tubule ending in a collecting duct with several other nephrons. · Respiratory acid-base disorders are caused by alterations of PaCO2, without any change of the amount of titratable base in the extended ECV (BE is zero). · Respiratory acidosis is an acidosis with a PaCO2 above 6.4 kPa or 48 mmHg at rest · Respiratory alkalosis is an alkalosis with a PaCO2 below 4.4 kPa or 33 mmHg at rest. · Titratable phosphate acidity in the daily urine is the amount of base (mmol) needed to titrate an acidic urine (phosphoric acid) back to the pH of plasma and glomerular filtrate (pH = 7.4 and PCO2 of 5.3 kPa). · Tubular reabsorption is the movement of water and solute from the tubular lumen to the tubule cells and to the peritubular capillary network · Tubular secretion represents the net addition of solute to the tubular lumen. This paragraph deals with 1. Proton concentration & pH, 2. Buffer capacity and the bicarbonate buffer , 3. Buffer capacities, 4. From the H-H-equation to the acid-base chart, 5. Extended ECV & Base excess, 6. Metabolic acid-base production, 7. Renal acid-base control, 8. Intracellular buffers. 1. Proton concentration and pH Normally, the [H+] of arterial plasma of humans at rest is maintained by the lungs and kidneys within the range of 40 ± 5 nM, corresponding to a pH of 7.36-7.44. The [H+] of the arterial plasma is also maintained during strenuous conditions and diseases: 16-160 nM or pH 6.8-7.8 (Fig. 17-1). Proton concentrations in nM differ by a factor 10-6 from the normal standard-[Na+], which is around 140 mM. A pH of 6.8-6.9 is not sustainable for long, and the patient is dying in a state of coma. In contrast, a healthy athlete can survive a pH of 6.85 following supramaximal exercise with headache as the only consequence. A pH approaching 7.8 implies dissociation of all buffer proteins in order to produce H+ (Eq. 17-7), and negative albumin charges capture Ca2+, so the extracellular Ca2+ falls and the patient dies in tetanic cramps including laryngeal spasms (Fig. 17-1). Tetanic cramps are spontaneous prolonged or continuous muscular contractions (for explanation see later) Fig. 17-1: Relationship between pH and the proton concentration 2. Buffer capacity and the bicarbonate buffer The acid-base buffer capacity of a system is defined as the amount (mmol) of strong acid or base added to one litre (l) of the system (ie, mmol per l or mM) in order to change the pH one unit The importance of the CO2/bicarbonate buffer is easily realised when comparing the addition of one mmol of strong acid to one litre of a closed and an open system with 24 mM bicarbonate each (pH 7.4; PCO2 5.3 kPa). Fig. 17-2: The CO2/bicarbonate buffer working in a closed and an open system. In the closed system, the base concentration is reduced by 1 mM to 23, and the acid concentration is increased by one mM, because the reaction is shifted towards formation of CO2 causing a high PCO2 (Fig. 17-2). Accordingly, the acid concentration is now: ([0.225 × 5.3] + 1) = 0.225 × newPCO2. Thus the newPCO2 is 2.19/0.225 or 9.73 kPa. The pH of the closed system is changed to 7.02 (Fig. 17-2). The buffer capacity is 1/(7.4 - 7.02) = 2.6 mM per pH unit, which is negligible. In an open system such as the body, the ventilation simply eliminates excess carbon dioxide and the PCO2 is kept constant: 5.3 kPa or 40 mmHg. The chemoreceptors are bathed in extracellular fluid. Any rise in the PCO2 of the extracellular fluid is sensed by the chemoreceptors and releases a proportionate rise in ventilation. Thus, the new pH is 7.38 (Fig. 17-2). The buffer capacity is at least: 1/(7.4 - 7.38) = 50 mM per pH unit, which is an essential capacity (see Table 17-1) The buffer capacity of a system is already defined as the amount of strong acid or base added to one litre (l) of the system in order to change the pH one unit The most important buffer system for the regulation of [H+] in extended ECV is the carbonic acid (CO2)/bicarbonate system (82%), although its pK is 6.1 and not so close to ideal as for the primary/secondary phosphate system (Table 17-1). The CO2/bicarbonate buffer is distributed in the extended ECV (up to 15 l of CO2 is bound as bicarbonate). Its buffer capacity is high, since PaCO2 is rapidly maintained normal by respiration, and the kidneys control bicarbonate excretion.
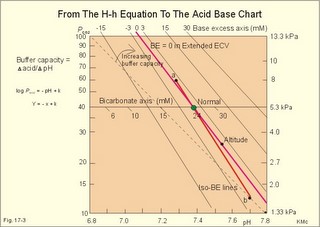
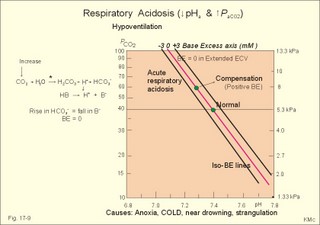
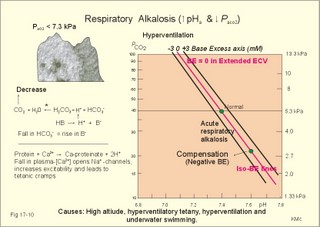
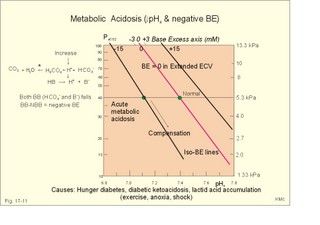
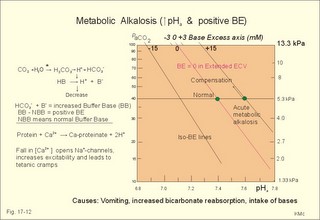
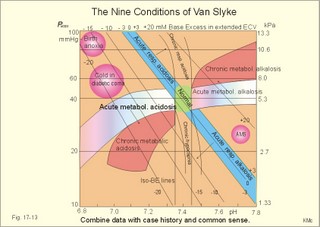
Of the non-carbonic buffer bases (18%), the haemoglobin has the dominating buffer value with 9 mM per pH unit in the extended ECV, leaving only about 2 mM per pH unit for proteins (essentially albumin) and phosphate (Table 17-1). 4. From The H-H-equation to the Acid-Base Chart The three variables in the final Henderson-Hasselbalch equation (pHa, PaCO2, and actual bicarbonate in Eq. 17-6) are easy to handle. Rearrangement of Eq. 17-6, with constant bicarbonate concentration, shows the following: log PaCO2 = - pH + k or y = -x + k, where k stands for three constants (6.1 + log [constant bicarbonate] - log 0.03). Thus, y is a linear function of x, and the slope of the line is -1. This slope is called an iso-bicarbonate line, and the slope reflects the buffer capacity (rise in carbonic acid per pH unit) of the bicarbonate buffer in water. Siggaard-Andersen has developed a double logarithmic plot, where factor 10 has the same distance (decade equidistant). The pH is chosen as the abscissa (pH = 6.8 - 7.8) and log PaCO2 is the ordinate (10-100 mmHg in Fig.17-3). Fig. 17-3: The pH-log PaCO2 plot (decade equidistant). All points on each iso-bicarbonate line in the plot with a slope of -1 have the same bicarbonate concentration. An example is the iso-bicarbonate line relating pH and log PaCO2 in a pure bicarbonate solution in water (15 mM) - shown by a stippled line with the slope -1 in Fig. 17-3. Here the bicarbonate concentration is constant (included in k) namely 15 mM in all points. Such iso-bicarbonate lines are pure mathematics. When two variables are given, the third variable is readable from the plot (or easy to calculate according to the equation). Originally, the equilibration line for a blood sample was determined by equilibration with high and low PCO2 as shown by the points a and b, crossing a bicarbonate axis in mM running through PaCO2 40 mmHg (= 5.3 kPa in Fig 17-3). The actual bicarbonate concentration is readable at this bicarbonate axis. As an example, let us assume that the point b, characterises the actual pH and PaCO2 measured. An iso-bicarbonate line is drawn with the slope -1 (use a piece of folded paper) and the actual bicarbonate for the point b is read on the bicarbonate axis to be 15 mM. This is simply a fast geometrical calculation Adding the non-carbonic buffers of whole blood (thick red line between a and b in Fig. 17-3) or of extended ECV (purple line) to a pure bicarbonate solution in water, naturally increases the buffer capacity. These lines are therefore steeper than the iso-bicarbonate lines (-1). The most important of the non-carbonic buffers is haemoglobin in the red cells (Table 17-1). The actual bicarbonate concentration is dependent of variations in PaCO2. When PaCO2 increases, carbonic acid is buffered by non-carbonic buffers causing a rise in actual bicarbonate (see the two interrelated reactions in Principles). The elevated actual bicarbonate is interpretable as a metabolic alkalosis, also when a respiratory acidosis is the real cause. The interpretation is a serious error Accordingly, we have to look for a better index to separate metabolic from respiratory acid-base disturbances The steepest equilibration line illustrates the buffer capacity for whole-blood buffers (Fig. 17-3 a to b). The slope of an equilibration line for all the buffers in the extended ECV is slightly smaller than that of whole-blood, because the whole-blood buffers are diluted in the large extended ECV (Fig. 17-3, purple line). Base Excess (BE) of the extracellular fluid (extended ECV) is calculated as the concentration difference of buffer bases in the actual sample and in the same sample following equilibration to standard conditions (pH 7.4, PaCO2 5.3 kPa or 40 mmHg and well oxygenated). A healthy person with these actual values for pH and PaCO2 must have a BE of zero. As PCO2 rises due to hypoventilation the [bicarbonate] rises, but BE of the extended ECV remains zero. The slope of the BE- zero line depends primarily on the concentration of haemoglobin, less on the plasma protein concentration, and minimally on the effect of inorganic phosphate (Table 17-1). The position of the BE-line depends on the BE: adding strong acid shifts the line to the left, and adding base shifts the line to the right. The series of almost parallel iso-BE-lines, with negative and positive values around the BE-zero line, has been determined experimentally. Each iso-BE-line is an equilibration line for a standard person in a given metabolic condition. The iso-BE-line zero depicts the condition with normal concentration and buffer capacity of buffer bases, and the other almost parallel lines represent abnormal conditions with either positive or negative base excess. With pH, and PCO2 measured, it is easy to read the precalculated base excess from the base excess axis at the top of the acid-base chart (Fig. 17-13). The extended ECV is the extracellular fluid volume plus the red cells. An abstraction is to think of all red cells distributed in the total extracellular fluid volume. A useful model of the extended ECV is an arterial blood sample diluted three folds with its own plasma (2+1). The BE is independent of PaCO2, since any change in PaCO2 implies opposite molar changes of the bicarbonate and the non-carbonic buffer concentrations. Hereby, there is no change in BE. Thus, the base excess is constant (normally equal to zero) during acute changes in PaCO2 by hyper- or hypoventilation. The buffer capacity of the carbon dioxide- bicarbonate buffer is high, since respiration and bicarbonate excretion by the kidneys rapidly maintain PaCO2. The base excess is a key variable in diagnosing metabolic disorders. The base excess tells the whole story, whereas the bicarbonate buffer only tells part of the story (Table 17-1) - and sometimes in error. This is what makes Base Excess the diagnostic choice. BE alterations from zero are therefore used to diagnose metabolic, as opposed to respiratory acid-base disorders. Ole Siggaard-Andersen also introduced the term 'concentration of titratable hydrogen ion' as an alternative to base excess. The 'Titratable Hydrogen Ion concentration Difference' can be shortened to THID, which is equal to base excess. The 2 terms simply represent the same concentration difference from normal in mM, but with opposite signs. Base excess is discharged, since the essential variable is not a base and in reality is not always an excess. Hence, from now on THID is used instead of base excess. THID in normal subjects is equal to zero, positive in primary, metabolic acidosis, and negative in primary, metabolic alkalosis. 6. Metabolic acid-base production The daily metabolic production of carbon dioxide is up to 24 mol. The CO2 is a potential acid as H2CO3, and because the lungs eliminate it, it is called a volatile acid. The hepatic production of non-volatile acid depends upon its daily amino acid load from intestinal absorption including amino acids from the daily degradation of intestinal mucosal cells. Persons on a normal mixed diet produce up to 100 mmol fixed or non-volatile acids daily mainly from amino acids (plus organic acids as uric acid, phosphoric acid, and minimal amounts of HCl and sulphuric acid) Persons on a mixed diet absorb approximately 50 mmol organic bases daily (RCOO- in Table 17-2). A maximum of 20 mmol of these organic anions is excreted in a 24-hour urine, because at least 30 mmol react with H+ produced by the liver. As stated above, the hepatic oxidation of sulphydryl groups in amino acids and hydrolysis of phosphate esters from lipids produces 100 mmol H+ daily, of which 60-70 mmol is excreted in a 24 hour urine (Table 17-2).
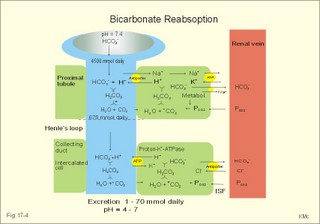
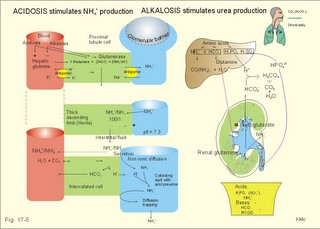
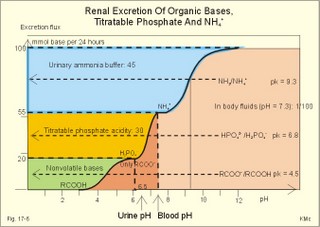
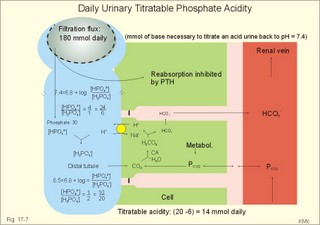
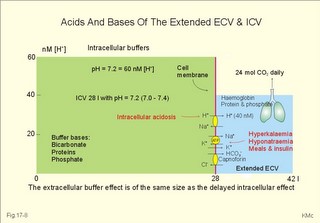
As stated above, the remaining 30 mmol of metabolic H+ is eliminated daily by oxidation of 30 mmol organic bases from the gut (RCOO-). About 30 mmol NH4+ is excreted in the daily urine, but the excretion is controlled during acid-base disorders. During acidosis, the high [H+] stimulates hepatic glutamate- and renal NH4+ -production, and the renal NH4+ -excretion increases towards 400 mmol daily. During alkalosis the urea production accounts for the nitrogen elimination and only negligible amounts of NH4+ is excreted Diets rich in vegetables and fruits generate non-volatile bases (salts of organic acids such as oxalate and citrate), which must be metabolised or excreted im the kidneys. In the body only amino acids are metabolised to bases (ammonia). Urinary excretion of the base form of ammonia takes place only in extremely basic urine. Normally, there is no non-volatile acid-base production from carbohydrates or lipids. The kidneys prevent the loss of enormous amounts of bicarbonate in the urine, produce the most important urinary buffer (NH3/NH4+ -buffer), and excrete the titratable phosphate acidity. - The free H+ is not a major issue, since even acid urine with a pH =5 only eliminate 1/100 mmol per l The details of paragraph 7.1 to 7.3 are best understood following a careful study of Chapter 25. Normally, the daily filtration flux of bicarbonate amounts to 4500 mmol (ie, filtration of 180 l of plasma with a mean concentration of 25 mM). Most of the filtered bicarbonate flux is reabsorbed already in the proximal tubules, where the luminal membrane contains a Na+-H+-antiporter (Fig. 17-4). The bicarbonate reabsorption is accomplished by means of H+-secretion. Most of the H+ secreted in the proximal tubules is derived from the Na+-H+-exchange through the antiporter. When the tubular fluid reaches the collecting ducts an important H+-secretion is mediated by a proton-K+-ATPase in the intercalated cells (see below). Any change in the filtered bicarbonate flux is matched by a similar change in proximal bicarbonate reabsorption (ie, glomerulo-tubular balance). A change in Na+ - homeostasis alters the bicarbonate reabsorption secondarily. The Na+-K+-pump in the basolateral membrane provides the energy for the secretion of H+ into the tubular fluid. This secretion serves to reabsorb the filtered bicarbonate, which is thus not excreted in the urine. The daily tubular secretion of H+ is enormous, because we excrete 70 mmol of non-volatile acid and also have to match almost all of the total filtration flux of bicarbonate. At the brush border of the proximal tubule cell, carboanhydrase (CA) catalyses the reaction, so CO2 is formed and can enter the cell easily by diffusion (Fig. 17-4). Fig. 17-4: Reabsorption of bicarbonate in the proximal and distal parts of the nephron. Also within the cell CA facilitates the production of (H+ + HCO3-). For each bicarbonate produced in the cell from the CO2 of the tubular fluid, one bicarbonate ion diffuses to the interstitial phase and the renal venous blood back to the body (Fig. 17-4). The cells of the thick ascending limb of the Henle loop also reabsorb bicarbonate by the same mechanism as in the proximal tubule The small residue of bicarbonate enters the distal tubules, where it is reabsorbed almost totally through a special mechanism independent of Na+ . In the intercalated cells of the collecting ducts, the reabsorption is dependent on a proton-K+-ATPase (Fig. 17-4). The bicarbonate ion crosses the basolateral cell membrane in exchange of chloride through a chloride-bicarbonate antiporter. - This special mechanism is most likely ineffective in distal renal tubular acidosis. Acidosis, which involves the intracellular space and stimulates production of proton-K+-ATP-ases, also favours H+ -secretion. Hereby, bicarbonate reabsorption is stimulated, whereas alkalosis inhibits bicarbonate reabsorption by the opposite mechanisms. Aldosterone stimulates the proton-K+-ATPases of the intercalated cells and the Na+-reabsorption/ K+ -secretion of the principal cells. Both effects favour H+ -secretion and thus bicarbonate reabsorption. As stated above, the most important urinary buffer is NH3/NH4+. This is because the synthesis of NH3/NH4+ in the tubule cells is controlled by the acid-base status of the body. Acidosis stimulates NH4+-production from renal glutamate, whereas alkalosis stimulates hepatic urea production from hepatic glutamate In a normally dieting adult person the amino acid load is from 90 g of protein (16% nitrogen) daily. The (90* 0.16)= 14.4 g nitrogen (1 mol) corresponds to 1000 mmol of NH3 /NH4+. According to the equation: 2 NH4+ + 2 HCO3-® CO(NH2)2 + CO2 + 3 H2O One mol of nitrogen daily produces 500 mmol urea, which is equal to the typical urinary urea excretion. The daily urea filtration flux is 900 mmol (5 mM * 180 l of plasma each day). The degree of reabsorption of the water-soluble urea depends upon the tubular flow rate Under normal conditions only a small amount of nitrogen is used to produce hepatic glutamate, which is an excellent atoxic ammonia store that can transfer ammonia to the proximal tubules of the kidneys in cases of acidosis. Fig. 17-5: Glutamate metabolism and renal ammonia production. The renal handling of ammonia and the excretion by diffusion trapping is shown. In the proximal tubules of the kidneys, renal glutamate produces NH4+ and a-ketoglutarate. One molecule of NH4+ is produced by deamination of one glutamine molecule by the enzyme, glutaminase, and a second by oxidative deamination of glutamic acid forming a -ketoglutarate that is metabolised. The NH4+ in the proximal tubule cells is in equilibrium with minimal amounts of NH3 at the relatively low pH. The NH4+-secretion into the tubular fluid makes use of the Na+-H+-antiporter, where NH4+ substitutes H+. The NH4+ passes with the tubular fluid to the thick ascending limb of the Henle loop, where a major portion is reabsorbed and accumulated in the interstitial fluid (Fig. 17-5). Secretion of NH4+ in the collecting ducts involves a special mechanism. The NH3 is lipid soluble and easily passes any membrane, so it reaches the tubular fluid of the collecting ducts and form NH4+ at the low pH (Fig. 17-5). The charged molecule cannot pass the membrane and it is trapped in the tubular fluid and eliminated in the urine. This diffusion trapping of charged molecules such as NH4+ is called non ionic diffusion - a general elimination principle for many charged metabolites and drugs. Excretion of NH4+ reduces the excretion of other positive ions. The a -ketoglutarate is metabolised into bicarbonate. Bicarbonate of the extracellular fluid reacts with H+ from hepatic phosphoric and sulphuric acid to form carbon dioxide and water. The H2PO4- (and a minimal amount of SO42-) is excreted in the urine. On a mixed diet the production and excretion of non-volatile acids and bases results in a net excretion of acids equal to the daily net production of non-volatile acids. With a urine pH of 6.5, organic acids such as lactic acid, b-hydroxybutyric acid, pyruvic acid etc., are present in the base form (RCOO- of Fig. 17-6). Most of the phosphoric acid is H2PO4-, and almost all ammonia is in the NH4+ form. Fig. 17-6: Excretion flux for organic bases (RCOO-), titratable acid (H2PO4-), and NH4+ in normal daily urine. This is not so in an alkaline urine. At a urinary pH of 8, there is 5% NH3 of the total, just as in ileum and colon, where the pH is also 8. The high pK (=9.3) of NH3/NH4+ has the consequence that in gastric juice with a pH of 1, the (pH-pK)- difference is -8.3, so virtually all ammonia must be NH4+. Even in body fluids with a pH of 7.3 the NH3/NH4+ ratio is 1/100. 7.3 The titratable phosphate activity The dominating buffer system in the urine is secondary/primary phosphate. This is because of its urinary concentration and of its pK (6.8) being close to urinary pH (6.5). A healthy person has a renal filtration flux of phosphate of 180 mmol daily (1 mM*180 litres of plasma filtered daily). Phosphate is a threshold substance, which is reabsorbed, in the proximal tubules, where parathyroid hormone (PTH) inhibits phosphate reabsorption (Fig. 17-7). With 30 mmol left in the tubular fluid, the secondary/primary phosphate-ratio is 24/6 mmol as calculated in Fig. 17-7. Secretion of H+ during the passage of the fluid through the renal tubules converts HPO42- to the acid form, H2PO4-. Thus, in the final urine, the base/acid-ratio is 10/20 (Fig. 17-7). Titratable phosphate acidity in the daily urine is the amount of base (mmol) needed to titrate an acidic daily urine back to the pH of plasma and glomerular filtrate (pH 7.4). Weak acids are not titrated, because they are minimally dissociated at pH 6.5 to 7.4. Normally, the titratable phosphate acidity is 30 mmol in a 24 hour urine. In our example above, the distal H+-secretion has titrated 14 mmol of HPO42- to H2PO4- (Fig. 17-7). Acidosis increases the urinary titratable phosphate acidity (towards 50 mmol daily) in order to get rid of the acid. Fig. 17-7: Titratable phosphate acidity produced in the distal tubules. The small amount of bicarbonate in the daily urine (zero to 3 mmol) hardly affects the measured titratable phosphate acidity, and the ammonia buffer is not titrated in acid urine The importance of the buffer capacity of the extended ECV, and that of the intracellular fluid is comparable in the majority of acute conditions, with extended ECV as the initial distribution volume and the intracellular fluid participating importantly after hours. This is because the alteration of the cellular transport processes takes time. The [H+] of the intracellular fluid volume (ICV) is higher than that of the extended ECV. The intracellular pH is precisely controlled in cells with different functions and needs, and the range of values is 7.0 -7.4 (Fig. 17-8). Fig. 17-8: The proton concentrations and buffer bases of the extended ECV and of the ICV. The buffer bases within the cells are proteins, phosphate and bicarbonate. The precise intracellular control is necessary for the pH-sensitive cellular processes with pH-optima for the enzyme systems. The active transport of H+ out of the cell is a coupled Na+/H+ -exchange. The energy for this exchange is delivered by the Na+-K+-pump, which maintains the Na+ -gradient across the cell membrane (Fig. 17-8). Carbohydrate- and K+-containing meals, insulin, hyperkalaemia, adrenaline and aldosterone stimulate the Na+-K+-pump. A bicarbonate-transport protein (capnoforin in the red cell and in many other cell membranes) transfers bicarbonate to the extended ECV by bicarbonate/chloride exchange (Fig. 17-8). In disorders with extracellular accumulation of carbon dioxide, CO2 diffuses rapidly into the cells. This causes a shift towards the right in Eq. 17-4. The intracellular buffers buffer the H+. Bicarbonate leaves the cells both directly via capnoforin and via other membrane channels (Fig. 17-8), whereby intracellular bicarbonate falls. Intracellular acid accumulation accompanied by hyperkalaemia may develop, if not compensated by the lungs. The K+-output follows the bicarbonate exit Non-volatile acid is also buffered intracellularly during metabolic acidosis by movement of H+ into the cell, where it reacts with proteins, phosphate and bicarbonate. During metabolic alkalosis movement of H+ out of the cells in exchange of Na+ (Na+-influx in Fig. 17-8) also buffers non-volatile base. Humans can suffer from 4 acid-base disorders: 1. Respiratory acidosis, 2 Respiratory alkalosis, 3 Metabolic acidosis and 4 Metabolic alkalosis. Acidosis (acidaemia) is defined as a disorder with pH in the arterial blood (pHa) less than 7.35, and alkalosis (alkalaemia or baseosis) is defined as a condition with a pHa larger than 7.45. Each of these two disorders has respiratory and metabolic forms. Respiratory acid-base disorders are caused by primary changes of PCO2, and compensated by altered renal excretion of acid in a matter of days. Metabolic acid-base disorders are caused by primary changes in BE, and compensated partially by the lungs in a matter of hours. The final correction of metabolic disorders is always renal and takes several days. Let us now return to the four primary acute disorders and their four chronic forms: is caused by hypoventilation (or breathing of CO2 containing air). Hypoventilation is associated with an impaired ability to eliminate CO2, whereby PaCO2 increases and the accumulated CO2 reduces the arterial pH. For each mol of bicarbonate produced, one mol of non-carbonic buffer base is eliminated, which means that Base Excess (BE) is unchanged zero (Fig. 17-9). The slope of the THID -zero line depicts the buffer-base capacity of the extended ECV. Any primary respiratory disorder is compensated renally over days. This is because the high intracellular [H+] increases the glutaminase synthesis and activity, the renal ammonia production, the urinary H+-excretion (mainly NH4+ but also H2PO4-), with a virtually complete reabsorption of filtered bicarbonate. Hereby, BE becomes positive during compensation (arrow in Fig. 17-9). Recall BE is now THID but with inverse signs, but for didactic purposes we use BE. Fig. 17-9: Acute respiratory acidosis with a base excess of zero, and its compensation. In severe cases of chronic CO2 accumulation, artificial ventilation is necessary. This is often the case in the terminal phase of chronic obstructive lung disease (ie, chronic bronchitis and emphysema). Other causes of respiratory acidosis are asthma, pulmonary cancer or tuberculosis, polio, drug overdose, anaesthesia, strangulation, near drowning and myasthenia gravis. 2. Respiratory Alkalosis is caused by hyperventilation. The hyperventilation is disproportionately high compared to the CO2 production, whereby the PaCO2 falls and the pH increases (Fig. 17-10). When the alveolar ventilation is doubled, the PaCO2 is halved. This is a typical reaction to high altitude. As the PIO2 falls with increasing altitude, the PaO2 eventually falls below 55 mmHg, which stimulates the chemoreceptors to hyperventilation (CO2 -wash-out). Other typical cases are the anxious patient during an attack of asthma or the hysterical hyperventilation in neurotic patients. These patients often experience tetanic cramps (see below) Hyperventilation before underwater swimming eliminates the CO2 stimulus and shifts the oxyhaemoglobin dissociation curve towards the left. Hereby, oxygen is bound firmly to haemoglobin. When the PaO2 falls below 30 mmHg (4 kPa), blackout and grey-out occurs. Loss of consciousness below water is often fatal. Fig. 17-10: Acute respiratory alkalosis and its compensation. For each mol of bicarbonate eliminated, one mol non-carbonic buffer base is formed, which means that THID is maintained at zero (Fig. 17-10). As long as no non-carbonic acid or base is added to the extracellular fluid volume, the extracellular base excess remains unchanged (zero) Acute respiratory alkalosis is compensated by increased renal excretion of bicarbonate, which is the result of decreased tubular H+ -secretion. This is because the low PaCO2 reduces the tubular H+-secretion, and the alkalosis inhibits formation and secretion of NH4+ . The renal mechanisms affect the production and activity of cellular enzymes and hormones, so it takes days to become effective. After a few days the renal compensation of the respiratory alkalosis is complete, and the pH is normal. This is called totally compensated respiratory alkalosis. - In cases of asthma-anxiety or hysteria with hyperventilation tetany, simple rebreathing from a bag cures the disorder within minutes. Dissociation of protein molecules occurs in all types of alkalosis in order to liberate H+. The dissociation leads to tetany: Protein + Ca2+ ® Ca-proteinate + 2 H+. The equilibrium dislocates towards the right in alkalosis. The falling extracellular [Ca2+] activates Na+-Ca2+-pumps and opens Na+-channels in the cell membranes of neurons, muscle cells and the myocardial syncytium. The Na+-influx reduces the membrane potential and increases the excitability of the tissues, which causes tetanic cramps (almost continuous muscular contractions). is caused by accumulation of strong acids in the extended ECV. Metabolic acidosis is diagnosed by negative base excess, because both types of buffer bases are reduced. In Fig. 17-11 both types of buffers and the total concentration of buffer bases of the extended ECV are clearly reduced and the BE is -15 mM. The iso-base excess-line (-15 mM) is steeper than the base excess-zero line, whereas the iso-base excess-line (+15 mM) is less steep than the BE-zero-line, due to the relative low pK-values of essential buffers. Strong acids accumulate, because of excess production or impaired H+ -excretion. Hunger (hunger diabetes), diabetic ketoacidosis, lactic acid accumulation or high protein intake with increased production of hydrochloric and sulphuric acid, cause excess production. Fig. 17-11: Acute metabolic acidosis: The base excess is reduced. The compensation is shown with an arrow. Impaired renal H+ excretion is related to increased loss of bicarbonate in the urine (due to renal failure). Diarrhoea causes acidosis by loss of bicarbonate with the faeces. Any loss of bicarbonate in the urine or faeces is equivalent to an addition of H+ to the extracellular fluid (Fig. 17-11). Lactic acidosis is caused by increased lactic acid production during exercise, shock, anoxia or following cardiac arrest. Another type of lactic acidosis is caused by decreased hepatic lactate metabolism - often drug-induced. Renal tubular acidosis is a damage of tubular cells caused by drugs or immunological reactions - or it may be inherited. The impaired H+ secretion reduces the tubular bicarbonate reabsorption. Kidney disease with destruction of a large number of nephrons reduces the tubular capacity to excrete H+ and NH4+ in the urine. The chloride-bicarbonate antiporter of the intercalated cells of the collecting ducts is probably ineffective (see above). The patient with metabolic acidosis suffers from dyspnoea (deep and frequent Kussmaull respiration). This hyperventilation is a respiratory compensation, which develops over hours as a reduction in PaCO2 (Fig. 17-11). This compensation is caused by the chemoreceptors, which are surrounded by the extended ECV and stimulated by its hydrogen ion concentration to react with hyperventilation. Acidosis shifts the oxygen dissociation curve to the right (ie, the Bohr effect), increasing the delivery of oxygen to the tissues. Acidosis stimulates K+ -loss from the cellular pool, because of K+ -efflux from the cells (Fig. 17-4). Chronic acidosis, however, inhibits 2,3-DPG production, which tends to shift the oxygen dissociation curve back towards the left When renal function is normal, K+ -loss from the cellular pool may lead to K+ -deficiency. When renal K+ -secretion is impaired, the cellular K+ -efflux may lead to hyperkalaemia. Oxygen enriched air is administered in cases of lactic acidosis with poor tissue bloodflow. Insulin must be given in diabetic ketoacidosis. A patient with metabolic acidosis and a base excess of -15 to -25 mM may have to be treated with bicarbonate infusion. The primary strategy is to eliminate the lack of base of the extended ECV. This strategy is accomplished by infusion of approximately X mmol bicarbonate (X= negative BE in mM multiplied by extended ECV). The extended ECV is approximately 20% of the body weight in kg or l. Careful monitoring of acid-base variables is necessary. Two errors are possible. Hours later, H+-ions from the cells enter the extended ECV and a further bicarbonate infusion is necessary. Correction of the primary disease (insulin to diabetic ketoacidosis) may in itself cure the acidosis by combustion of keto-acids to bicarbonate with the danger of overinfusion with bicarbonate. Continuous control of acid-base variables over days is therefore important. Rapid infusion of bicarbonate may be dangerous. A rapid decrease in [Ca2+] releases tetanic cramps (see alkalosis above). Administration of an overshoot of Na+-bicarbonate leads to volume expansion, pulmonary oedema, and a new equilibrium with too much CO2, which diffuses into the cells, worsening the intracellular acidosis, and causing bicarbonate efflux accompanied by hyperkalaemia. The final correction of a metabolic acidosis is always renal. is caused by a primary accumulation of strong bases in the extended ECV. Both the [bicarbonate] and the [non-carbonic buffer base] is increased, so the BE is increased (Fig. 17-12). The actual [bicarbonate] is often above 27 mM, which is the renal plasma concentration threshold for reabsorption. Above this threshold lots of bicarbonate is lost in the urine. Vomiting (loss of gastric acid and volume depletion), increased metabolism of lactate and citrate (turns into bicarbonate and water), and excessive intake of bases towards gastric ulcer can cause this form of alkalosis. Long-term use of thiazides and loop diuretics, K+-deficiency, and excess secretion of mineralocorticoid increase the H+-secretion in exchange for Na+ in the distal tubules. This increases renal bicarbonate reabsorption and leads to metabolic alkalosis. Fig. 17-12: Acute metabolic alkalosis: The base excess is increased. The hypoventilatory compensation is shown with an arrow. The hypoventilatory compensation reduces pH, but raises PaCO2 (Fig. 17-12). The compensation is never total, since the rise in PaCO2 and the fall in PaO2 in itself oppose the hypoventilation. The delayed renal correction increases bicarbonate excretion by reducing its reabsorption. This correction is also counteracted by the rise in PaCO2, which stimulates tubular bicarbonate reabsorption. The final renal correction of metabolic disorders takes several days. Careful monitoring with replacement of Na+ and K+ is essential, in order to improve the renal excretion of bicarbonate. In metabolic alkalosis the kidneys increase H+-secretion less than the bicarbonate filtration, whereby the bicarbonate excretion is increased. - Metabolic alkalosis combined with hypokalaemia and reduced ECV often has increased H+-secretion, whereby the bicarbonate excretion is reduced. Such cases must be treated with NaCl and KCl. Only rarely is it necessary to infuse acid, when deficits of NaCl, K+ and Mg 2+ are corrected. A patient with metabolic alkalosis is therefore rarely treated with infusion of acids. In the very few cases, the acid of choice is an ammonium chloride solution, which produce H+, when NH3 is used for carbamide (urea) production in the liver. Metabolic alkalosis is difficult to compensate by the body and difficult to treat. Each OH- has a molecular weight 17 times larger than H+, so OH- passes the membrane channels comparatively slowly in metabolic alkalosis compared to the H+ -transfer of metabolic acidosis. This results in delayed intracellular transfer and buffering of the alkalosis. Cerebral insufficiency is common in alkalosis, and the respiratory centre is depressed. Alkalosis displaces the oxyhaemoglobin dissociation curve to the left, impairing the delivery of oxygen to the tissues. Dissociation of protein molecules may lead to tetany. Protein anions bind Ca2+ during alkalosis, reduces the free serum [Ca2+] and triggers tetanic cramps (see explanation). These four primary acid-base disturbances and their four compensated or chronic types constitute, together with the normal condition, the nine van Slyke conditions (Fig. 17-13). Plotting the measured pH and PCO2 in the acid-base chart allows estimation of the base excess (BE), and combined with the case history, the correct diagnosis can be reached. Fig. 17-13: The Siggaard-Andersen acid-base chart with the 9 conditions of van Slyke (modified with permission). Please observe that each point on the chart can be reached in several ways. Van Slyke, who made the first apparatus to diagnose acid-base disturbances, emphasised the importance of the case history and common clinical sense. The importance is illustrated by the following three examples. The first example is a case of respiratory acidosis due to chronic obstructive lung disease (COLD in Fig. 17-13) and a metabolic acidosis due to diabetic coma. Without the case history it is difficult to diagnose the primary and secondary events in the development of the patients condition. The second example is a case of respiratory alkalosis due to acute mountain sickness (AMS), complicated by a metabolic alkalosis (AMS in Fig. 17-13), because the patient is loosing acid by vomiting. The third example is a serious case of birth anoxia with a combined respiratory and metabolic acidosis. In this case instantaneous intubation and oxygenation with 50-40-30 % oxygen saved the newborn. The case history, not the acid-base variables, is the only source to the sequence of events. High Altitude Acid-Base correction At high altitude, the Van Slyke Equation has to be modified with a correction factor such that the acid-base charts are suitable. That way an acid-base correction is performed to the same altitude of residence and not to sea level values, that would be an agression instead of a return to baseline values. See Full text · Acid (HB) is defined as a compound that can release a hydrogen ion or proton (H+), whereas a base (B-) can take up H+. The H+-concentration is symbolised as [H+] just as other concentrations: Eq 17-1: HB ® H+ + B-, where the dissociation constant (K´) is defined by: K´ = [H+]* [B-]/[HB]. · This is the mass action equation, which can be logarithmically rearranged to: Eq 17-2: pH = pK´ + log ([B-]/[HB]); The pK´ is equal to the pH, when the acid is 50% dissociated. · For the carbonic acid system: Eq 17-3: CO2 + H2O Û *H2CO3 Û H+ + HCO3- Eq 17-4: pH = pK + log([bicarbonate]/[dissolved CO2]). · This is the Henderson-Hasselbalch-Equation - a logarithmic rearrangement of the mass action equation. · The law of Henry: Dissolved CO2 (ml l-1 of blood) is in equilibrium with and equal to PCO2 multiplied by the solubility coefficient a according to Henrys law: [dissolved CO2] = a* PCO2 (for the definition and size of a for CO2 see Table 13-1): Eq 17-5: (0.51 × 1000 × PCO2 )/(760 × 22.22) = (PCO2 × 0.03) mM with PCO2 in mmHg.. One mmol of ideal gas equals 22.4 ml STPD and since CO2 deviates slightly from an ideal gas its molar volume is only 22.22 l per mol. All the constants combine to 0.03. With kPa as unit for PCO2 (760 mmHg equals 101.3 kPa): 0.03 × 760/101.3 = 0.225; with this new constant the dissolved CO2 is equal to: (PCO2 × 0.225) mM. · At normal ionic strength and temperature the pK is 6.1 Eq 17-6: pH = 6.1 + log([bicarbonate]/(0.03 × PCO2)) with PCO2 in mmHg or pH = 6.1 + log([bicarbonate]/(0.225 × PCO2)) with PCO2 in kPa. This (Eq 17-6) is the final Henderson-Hasselbalch-Equation. · Proteins dissociate during alkalosis: Eq 17-7: Protein + Ca2+ ® Ca-proteinates + 2 H+, and the equilibrium dislocates towards the right. Of all the proton-binding groups on proteins, the calcium-binding groups are only a minor fraction. I. A patient who has been vomiting for several days is in hospital with the following findings in a sample of arterial blood: pH = 7.60; Base Excess = +20 mM; and PaCO2 = 6.7 kPa (50 mmHg). Each of the following disorders and statements has True/False options: A. Compensated respiratory alkalosis. B. Metabolic alkalosis C. Hypoventilation D. Increased base deficit E. Increased bicarbonate concentration. II. The following five statements have True/False options: A. In connection with alkalosis the free serum [Ca2+] is reduced, as proteins liberate H+ and bind more Ca2+. B. Metabolic acidosis is diagnosed by a negative base excess (base deficit). C. The titratable acid of the urine is the amount of acid needed to titrate the acid urine to the pH of plasma. D. The treatment of a patient with acute metabolic alkalosis and a base excess of 20 mM is hypoventilation in a respirator. E. Acute Mountain Sickness often causes vomiting. A female patient, 25 years of age, is undergoing a routine examination at an outpatient ward. Her pH is 7.42, and PaCO2 is 5 kPa (37 mmHg). The patient is suffering from a disorder with acute attacks of anxiety, panic, dyspnoea and tetanic cramps. A week later the patient is admitted to the emergency ward of the hospital with severe hyperpnoea and tetanic cramps. She has abnormal blood gas tensions: PaCO2 2.7 kPa (20 mmHg), PaO2 14.6 kPa (110 mmHg), and pH is 7.64. 1. Read her Base Excess of the extended ECV at the routine check by the help of Fig. 17-13. 2. Read her Base Excess of extended ECV during emergency conditions by the help of Fig. 17-13. 3. What is the most likely diagnosis? A 56-year-old male with chronic bronchitis and emphysema suddenly developed respiratory failure during flying in a pressurised intercontinental plane. The cabins of commercial aeroplanes are pressurised to a PB of 80 kPa corresponding to 2000 m. The patient was bluish in colour and the cabin personnel gave him a mask for breathing with pure (100%) oxygen, which was continued until landing. The patient was then admitted to hospital. An arterial blood sample (without supplementary oxygen) revealed the following: PaO2 6.7 kPa (50 mmHg), PaCO2 8 kPa (60 mm Hg) and pHa 7.44. The saturated water vapour pressure at body temperature is 6.26 kPa. 1 Calculate PIO2 in the moist tracheal air of the patient, when he breathes the air of the pressurised cabin just before respiratory failure. Is the oxygen tension insufficient? 2 Calculate the PAO2 of the patient at the hospital assuming PACO2 to be equal to PaCO2 and RQ =1. 3. Read the base excess of the extended ECV by the help of Fig. 17-13 and describe the acid-base disorder. Two male persons with a normal arterial pH (pHa = 7.40) are in hospital. One person starts to vomit excessively, which increases his pH to 7.80. The other person, a fit athlete, does a cardiopulmonary exercise test, which reduces his pH to 7.00. 1. Calculate the three concentrations of H+ (in nmol l-1 = 10-9 mol l-1) at these conditions. 2. The two changes in pH from the normal pH = 7.4 are similar. Why are the related changes in H+ concentration not identical? 3. Describe other conditions with metabolic acidaemia and metabolic alkalaemia. Try to solve the problems before looking up the answers. · The pH of the intra- and extra-cellular body fluids is maintained within narrow limits by the co-ordinated function of the respiratory and the renal system. · Persons on a high-protein diet produce 50-100 mmol fixed or non-volatile acids and 24 mol of volatile carbonic acid daily. · Produced and ingested acid-base is normally excreted, so the acid-base balance is maintained. · The kidneys prevent loss of bicarbonate. Of the renally ultrafiltered bicarbonate (4500 mmol daily) we reabsorb 90% already in the proximal tubules, and hardly any bicarbonate is excreted in the urine. · Urinary buffers (ammonia and phosphate) are essential for the excretion mechanism of non-volatile acid. · Acidosis (acidaemia) is defined as a disorder with pH in the arterial blood (pHa) less than 7.35, and alkalosis (alkalaemia) is defined as a condition with pHa larger than 7.45. · Respiratory Acidosis is caused by hypoventilation (or breathing of CO2 containing air). Hypoventilation is associated with an impaired ability to eliminate CO2, whereby PaCO2 increases and the accumulated CO2 reduces the arterial pH. · Respiratory Alkalosis is caused by hyperventilation, which is disproportionately high compared to the CO2 production, whereby the PaCO2 falls and the pH increases. · Metabolic Acidosis is caused by accumulation of strong acids in the extended ECV. Metabolic acidosis is diagnosed by negative base excess, because both types of buffer bases are reduced. · A primary accumulation of strong bases in the extended ECV due to vomiting, increased metabolism of lactate/citrate and excessive intake of bases cause metabolic alkalosis. · Respiratory acid-base disorders are caused by primary changes of PCO2 , and are compensated by altered renal excretion of acid in a matter of days. · Metabolic acid-base disorders are caused by primary changes in Base Excess, and are compensated partially by the lungs in a matter of hours. The final correction of metabolic disorders is always renal and takes several days, because production and activation of cellular enzymes and hormones are involved. · Alkalosis dissociates protein molecules, which bind ionised calcium. The hypocalcaemia opens Na+ - channels, and the influx increases the excitability of neuromuscular tissues, which releases tetanic cramps. Astrup, P. and J.W. Severinghaus. "The history of blood gases, acids and bases." Munksgaard, Copenhagen, 1986. Koeppen, B M. Renal regulation of acid-base balance. Am. J. Physiol. 275 (Adv. Physiol. Educ. 20): S132-S141, 1998. Siggaard-Andersen, O. The acid-base status of the blood. Munksgaard, Copenhagen, 1974. Siggaard-Andersen, O & OH Gøthgen (1995) Oxygen and acid-base parameters of arterial and mixed venous blood. Relevant versus redundant. Acta Anaesthesiol Scand 39. Suppl 107, 21-27. Siggaard-Andersen, O and N Fogh-Andersen (1995) Base excess and buffer base (strong ion difference) as measure of a non-respiratory acid-base disturbance. Acta Anaesthesiol Scand 39. Suppl 107, 123-128. Paulev PE, Zubieta-Calleja GR. Essentials in the diagnosis of acid-base disorders and their high altitude application. J Physiol Pharmacol. 2005 Sep;56 Suppl 4:155-70 Full text
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Click here to introduce your comments or contributions